BAB 5 THERMOCHEMISTRY SCIENCE FORM 3 CIKGU ERNEY
BAB 5 : THERMOCHEMISTRY SCIENCE FORM 3 CIKGU ERNEY WATI BINTI ZAILANI SM SAINS MUZAFFAR SYAH, MELAKA

1. What is the process involved shown on the Diagram ? 2. How does the process work? 3. Does the process involve heat changes? https: //www. advancedsciencenews. com/wp-content/uploads/2017/02/chain 566778_960_720. jpg
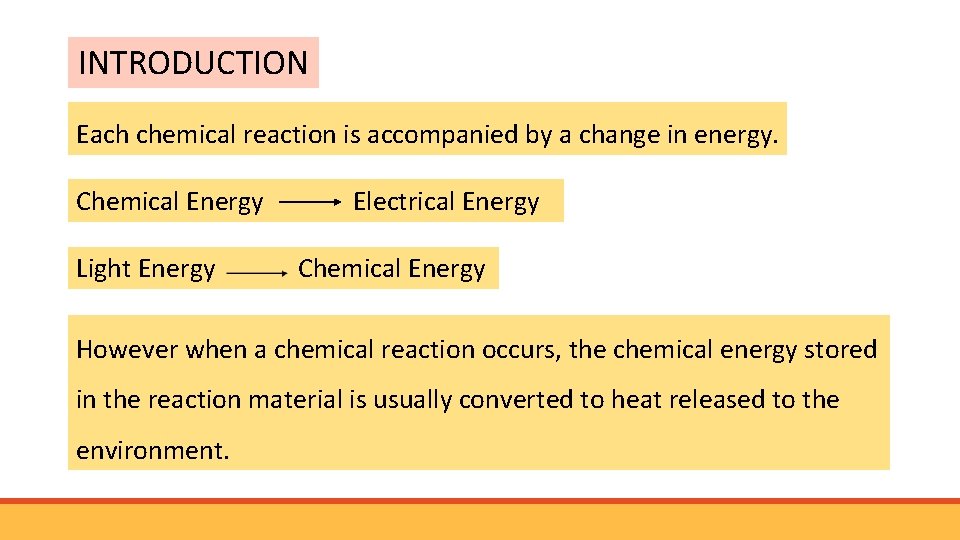
INTRODUCTION Each chemical reaction is accompanied by a change in energy. Chemical Energy Light Energy Electrical Energy Chemical Energy However when a chemical reaction occurs, the chemical energy stored in the reaction material is usually converted to heat released to the environment.
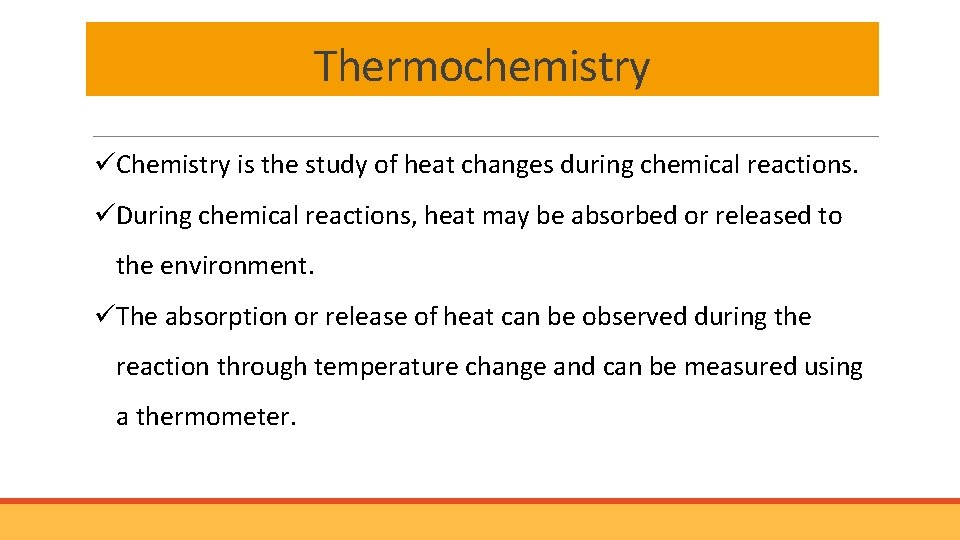
Thermochemistry üChemistry is the study of heat changes during chemical reactions. üDuring chemical reactions, heat may be absorbed or released to the environment. üThe absorption or release of heat can be observed during the reaction through temperature change and can be measured using a thermometer.
Types of chemical reaction Exothermic Reaction Endothermic Reaction
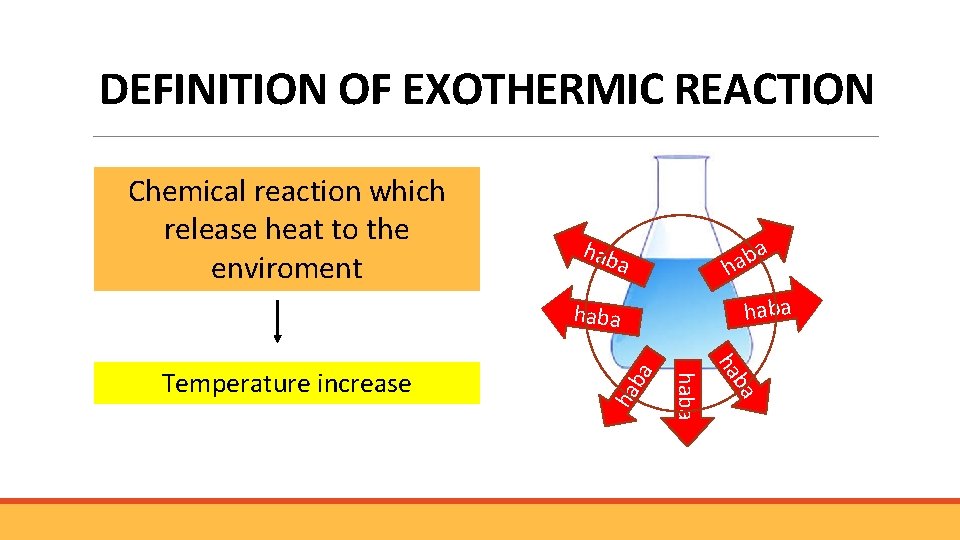
DEFINITION OF EXOTHERMIC REACTION Chemical reaction which release heat to the enviroment hab ba a haba ha haba Temperature increase ba haba
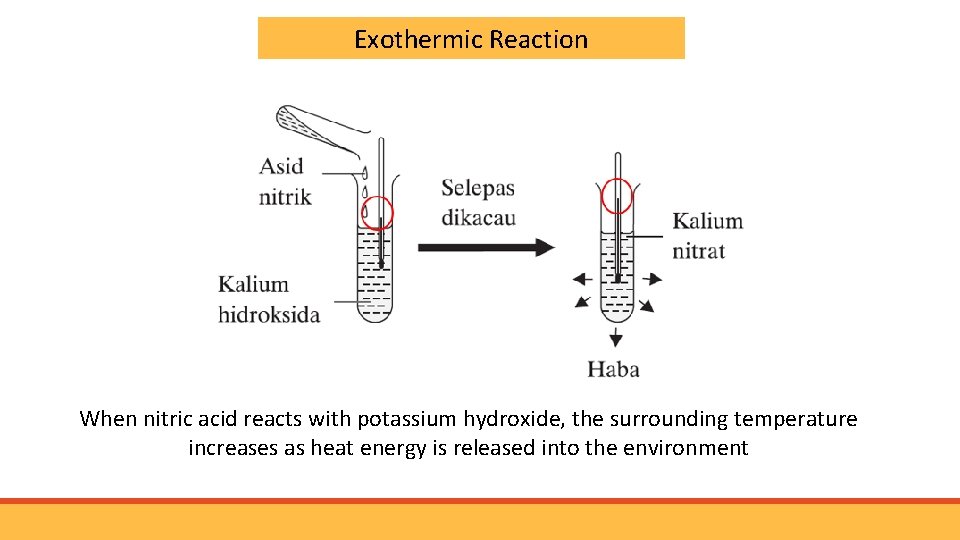
Exothermic Reaction When nitric acid reacts with potassium hydroxide, the surrounding temperature increases as heat energy is released into the environment
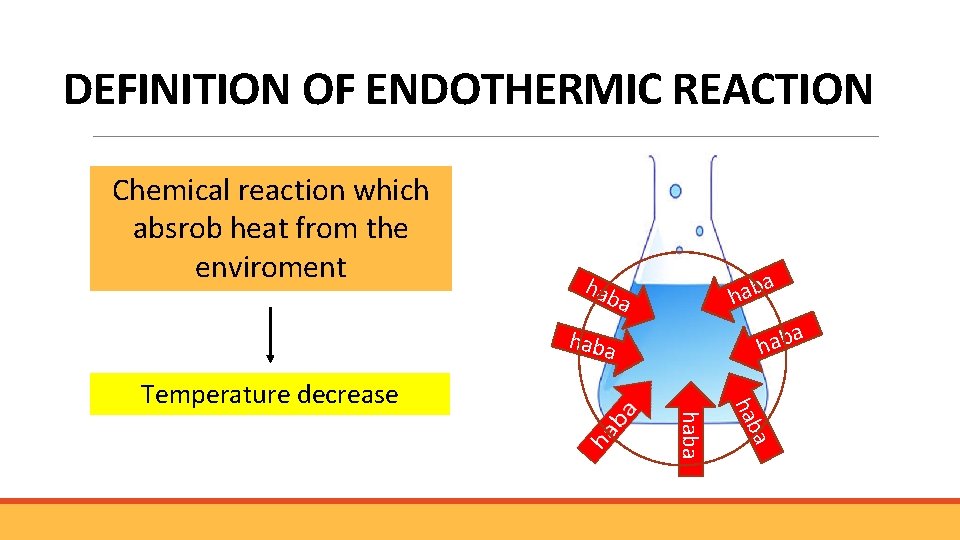
DEFINITION OF ENDOTHERMIC REACTION Chemical reaction which absrob heat from the enviroment a b a h hab a a haba a hab Temperature decrease
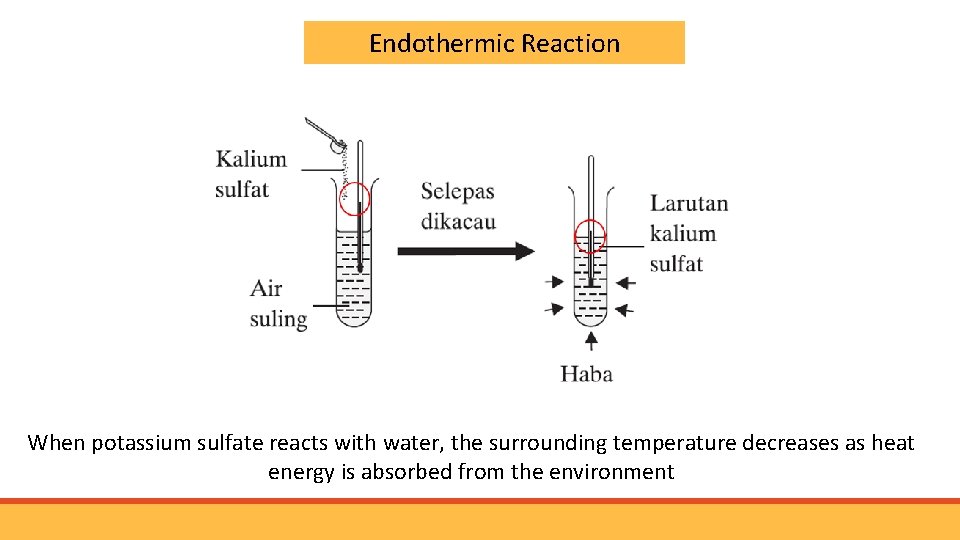
Endothermic Reaction When potassium sulfate reacts with water, the surrounding temperature decreases as heat energy is absorbed from the environment

Examples of Exothermic Reaction
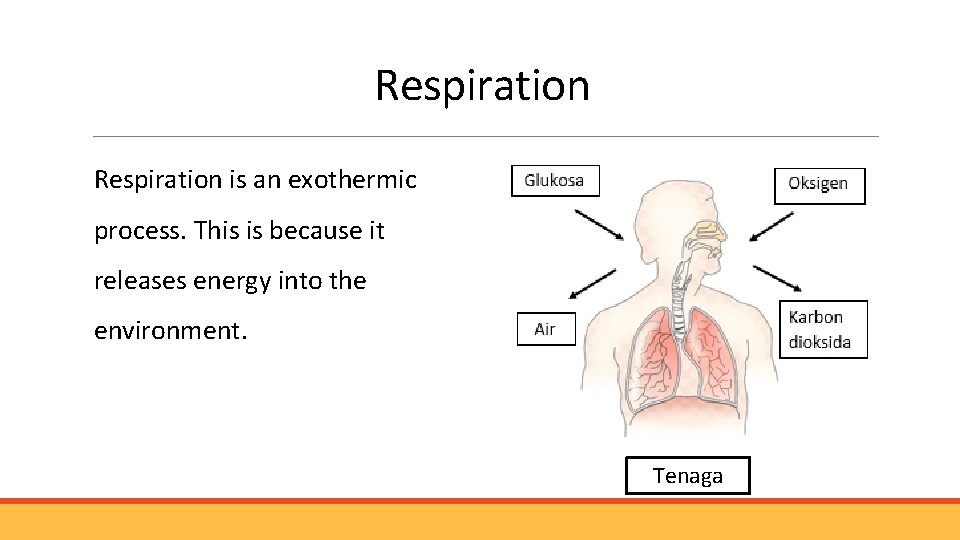
Respiration is an exothermic process. This is because it releases energy into the environment. Tenaga

Fire Craker Pembakaran bunga api membebaskan tenaga dalam bentuk tenaga haba ke persekitaran. https: //www. businessinsider. my/how-to-bake-acake-2019 -6
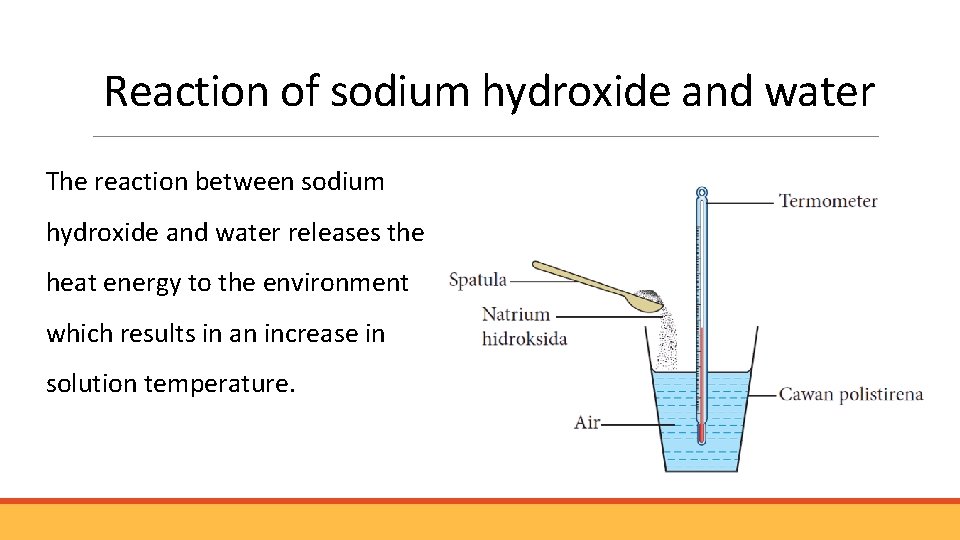
Reaction of sodium hydroxide and water The reaction between sodium hydroxide and water releases the heat energy to the environment which results in an increase in solution temperature.
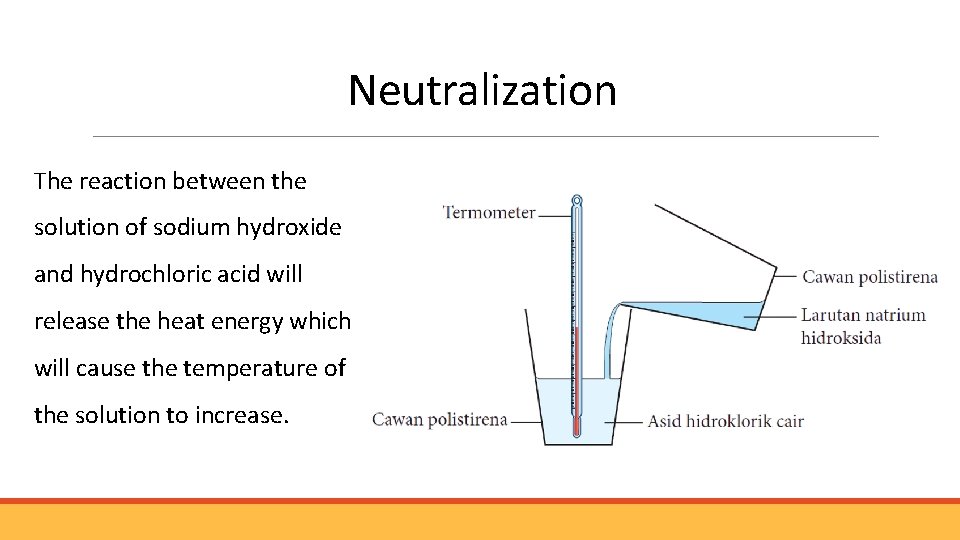
Neutralization The reaction between the solution of sodium hydroxide and hydrochloric acid will release the heat energy which will cause the temperature of the solution to increase.
Examples of Endothermic Reaction
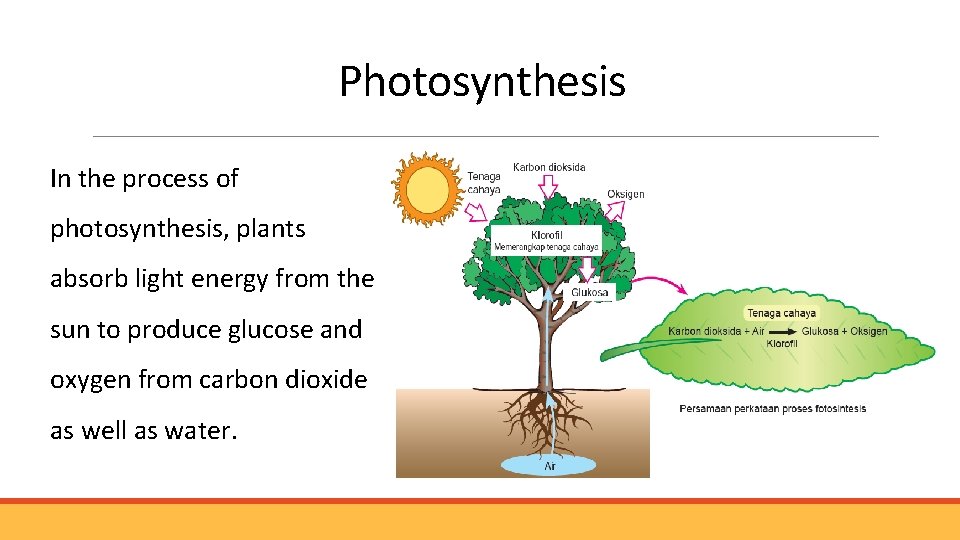
Photosynthesis In the process of photosynthesis, plants absorb light energy from the sun to produce glucose and oxygen from carbon dioxide as well as water.

Baking a cake • The method of baking a cake involves the use of heat. • This method uses an endothermic reaction in which the cake is cooked through the absorption of heat from the environment (oven). https: //www. businessinsider. my/how-to-bakea-cake-2019 -6
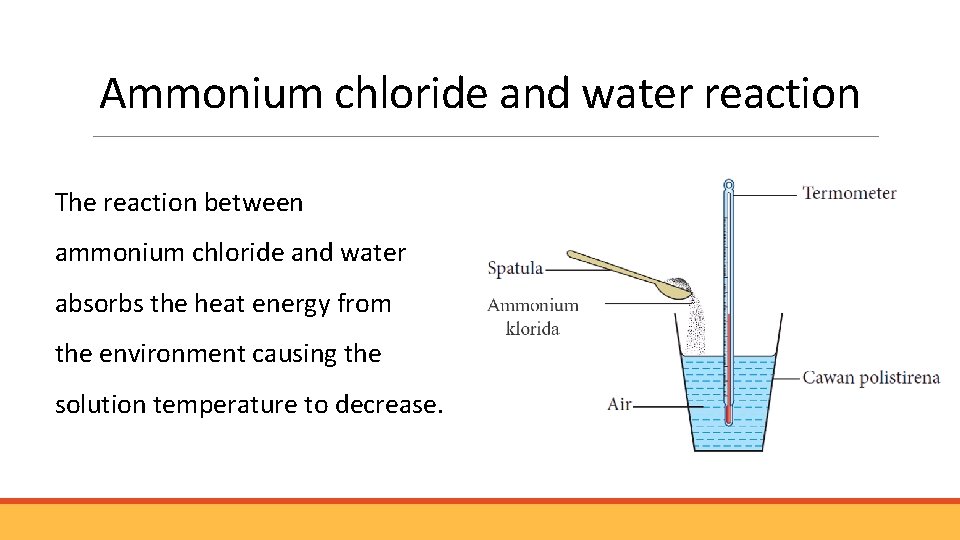
Ammonium chloride and water reaction The reaction between ammonium chloride and water absorbs the heat energy from the environment causing the solution temperature to decrease.

Using the Concept of Exothermic and Endothermic Reactions to Solve Problems in Daily Life
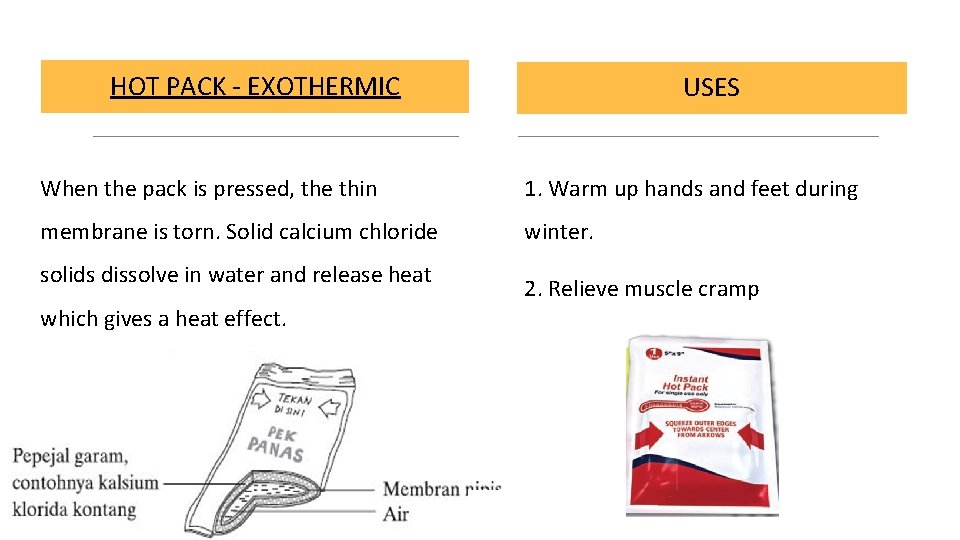
HOT PACK - EXOTHERMIC USES When the pack is pressed, the thin 1. Warm up hands and feet during membrane is torn. Solid calcium chloride winter. solids dissolve in water and release heat which gives a heat effect. 2. Relieve muscle cramp
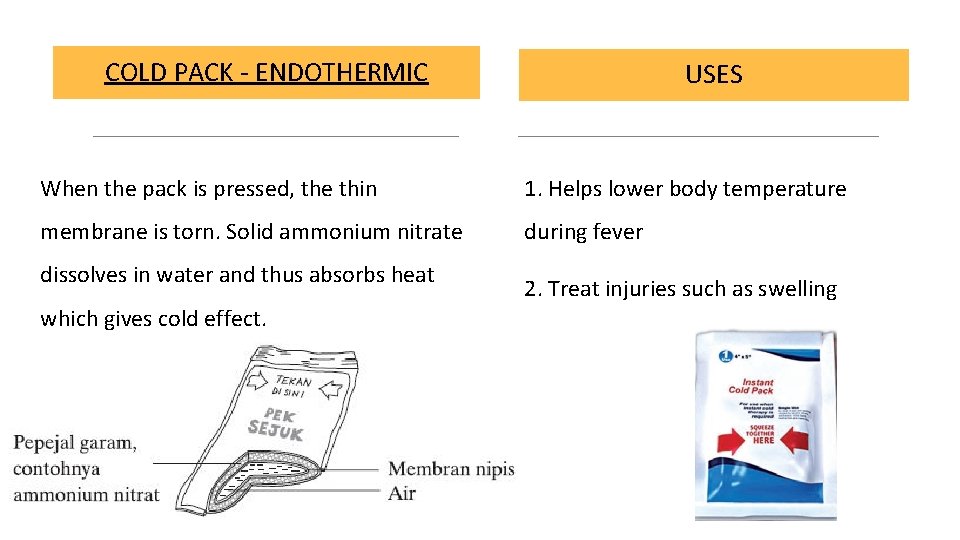
COLD PACK - ENDOTHERMIC USES When the pack is pressed, the thin 1. Helps lower body temperature membrane is torn. Solid ammonium nitrate during fever dissolves in water and thus absorbs heat which gives cold effect. 2. Treat injuries such as swelling
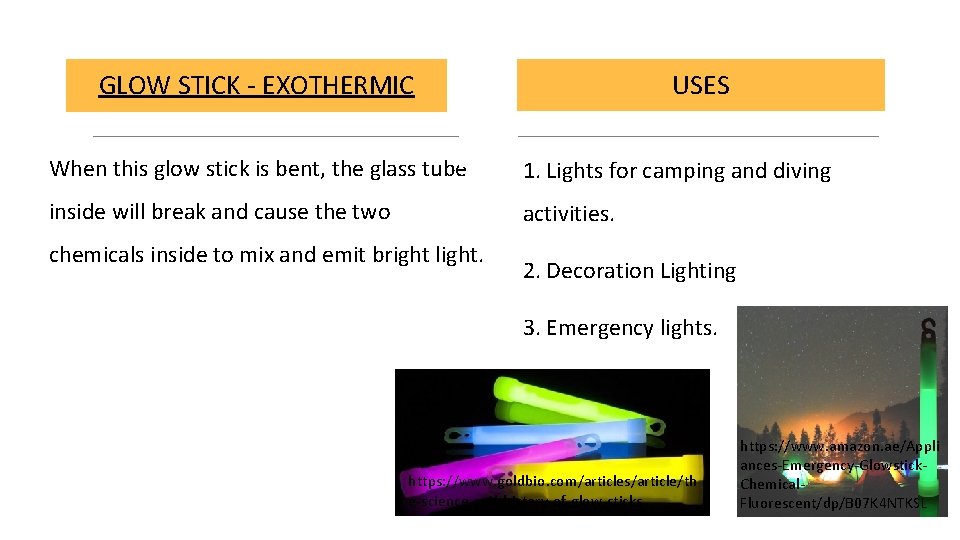
USES GLOW STICK - EXOTHERMIC When this glow stick is bent, the glass tube 1. Lights for camping and diving inside will break and cause the two activities. chemicals inside to mix and emit bright light. 2. Decoration Lighting 3. Emergency lights. https: //www. goldbio. com/articles/article/th e-science-and-history-of-glow-sticks https: //www. amazon. ae/Appli ances-Emergency-Glowstick. Chemical. Fluorescent/dp/B 07 K 4 NTKSL
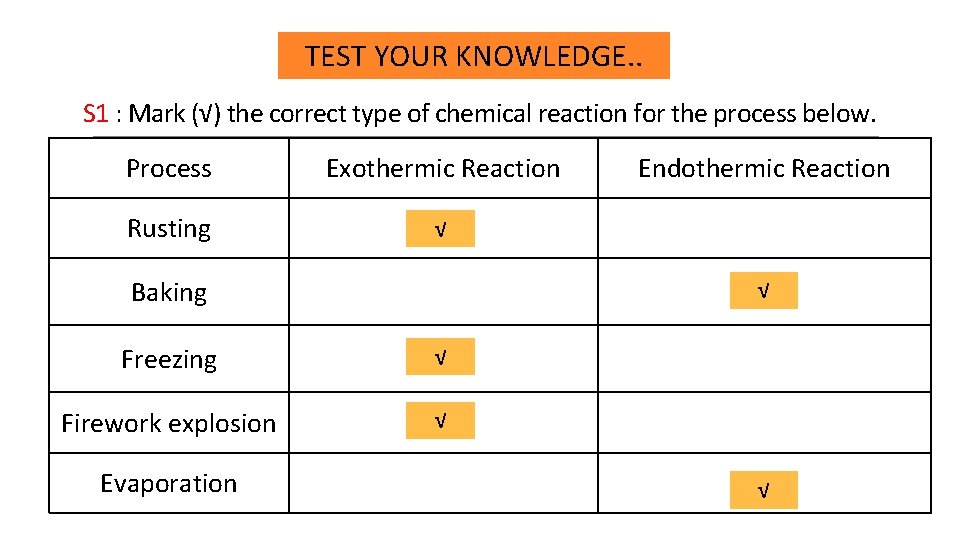
TEST YOUR KNOWLEDGE. . S 1 : Mark (√) the correct type of chemical reaction for the process below. Process Exothermic Reaction Rusting √ Baking √ Freezing √ Firework explosion √ Evaporation Endothermic Reaction √

S 2 : Build an appropriate i-Think map to illustrate the similarities & differences between exothermic and endothermic reactions.
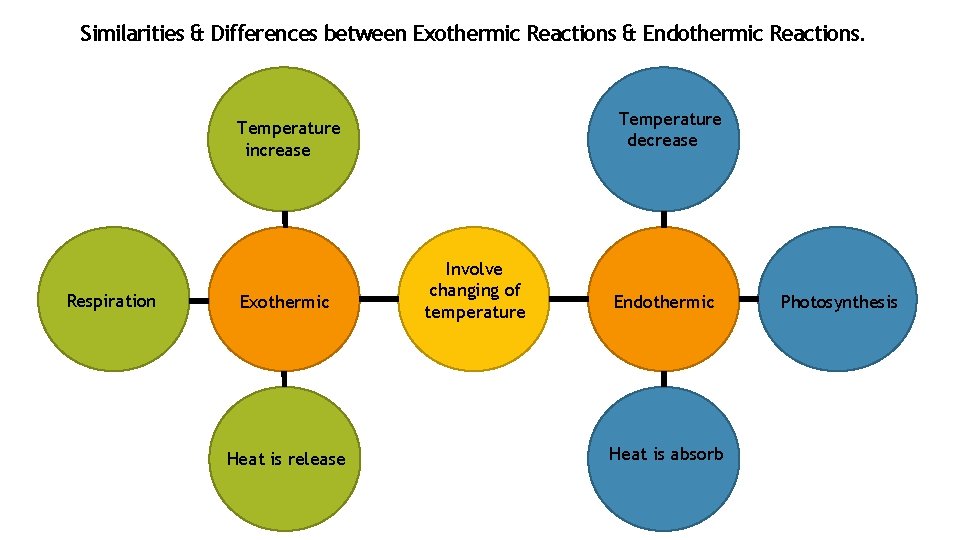
Similarities & Differences between Exothermic Reactions & Endothermic Reactions. Temperature decrease Temperature increase Respiration Exothermic Heat is release Involve changing of temperature Endothermic Heat is absorb Photosynthesis

Video https: //youtu. be/MTRKHr. Ac. LBc https: //youtu. be/subv-mmz. BYQ

- Slides: 28