B Sc Semester II Alkene Addition reaction Markownikoffs

B. Sc. Semester - II Alkene • • • Addition reaction Markownikoff’s rule : Statement Addition of Br 2 to ethene Addition of HBr to Propene Peroxide effect Free radical addition of HBr to Propene
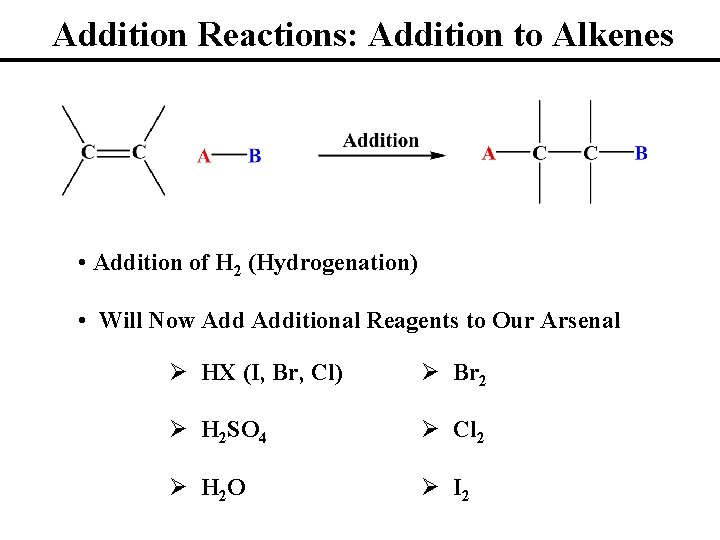
Addition Reactions: Addition to Alkenes • Addition of H 2 (Hydrogenation) • Will Now Additional Reagents to Our Arsenal Ø HX (I, Br, Cl) Ø Br 2 Ø H 2 SO 4 Ø Cl 2 Ø H 2 O Ø I 2
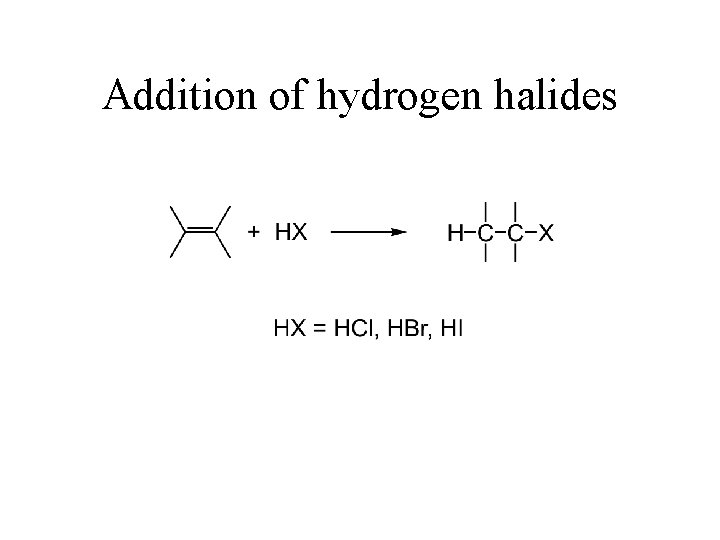
Addition of hydrogen halides

Addition of hydrogen halides Only 2 -chloropropane is formed
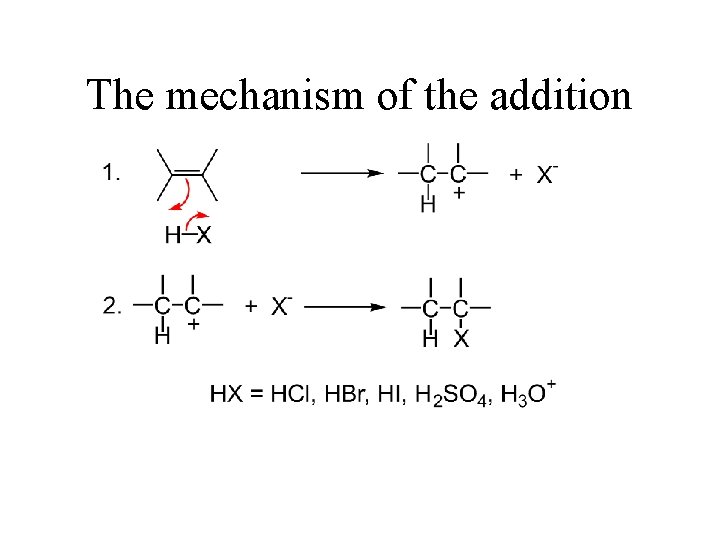
The mechanism of the addition

An example

Addition Reactions: HX to Alkenes • General Order of HX Reactivity: HI > HBr > HCl > HF • Usually Dissolved in Solvent (CH 3 CO 2 H, CH 2 Cl 2) • Addition of HCl not Generally Useful (Works w/ Silica Gel)
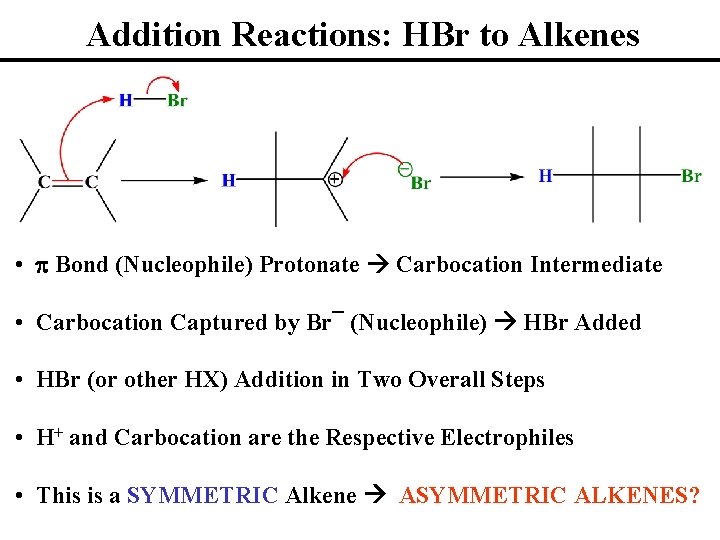
Addition Reactions: HBr to Alkenes • p Bond (Nucleophile) Protonate Carbocation Intermediate • Carbocation Captured by Br¯ (Nucleophile) HBr Added • HBr (or other HX) Addition in Two Overall Steps • H+ and Carbocation are the Respective Electrophiles • This is a SYMMETRIC Alkene ASYMMETRIC ALKENES?
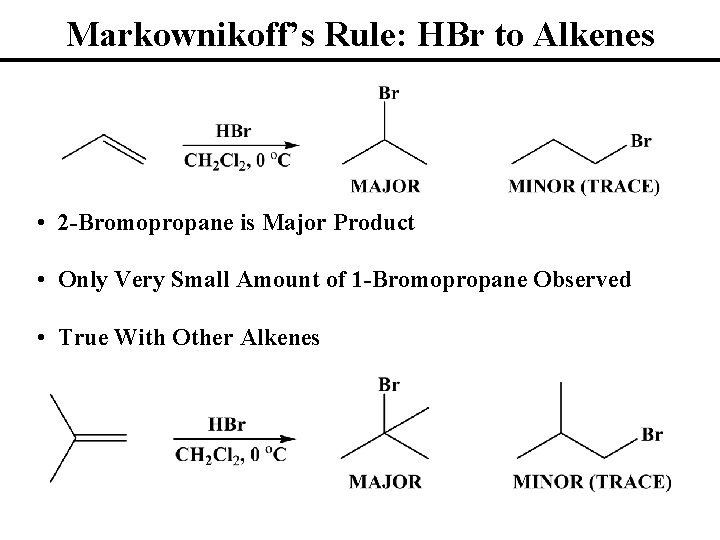
Markownikoff’s Rule: HBr to Alkenes • 2 -Bromopropane is Major Product • Only Very Small Amount of 1 -Bromopropane Observed • True With Other Alkenes
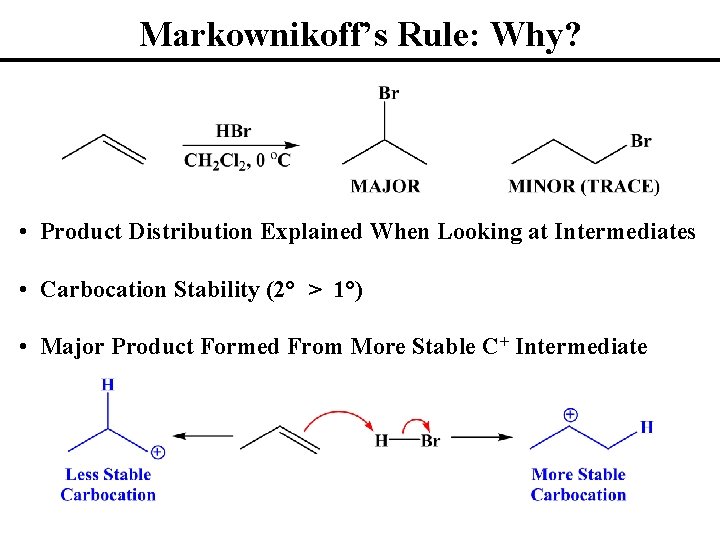
Markownikoff’s Rule: Why? • Product Distribution Explained When Looking at Intermediates • Carbocation Stability (2° > 1°) • Major Product Formed From More Stable C+ Intermediate
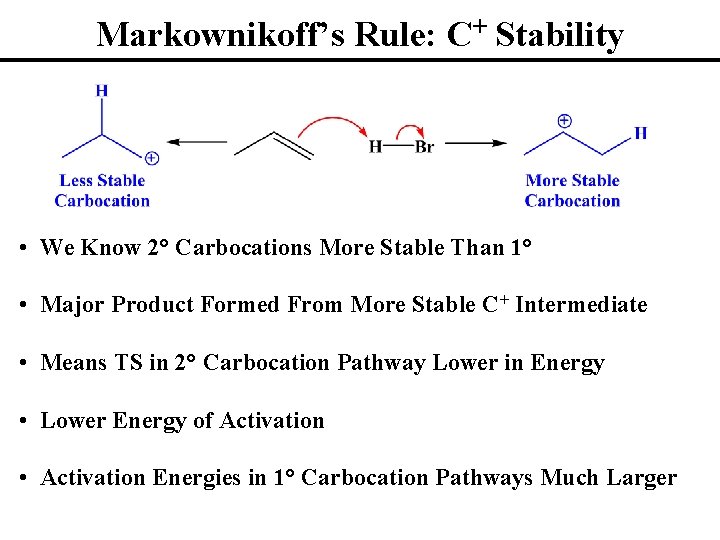
Markownikoff’s Rule: C+ Stability • We Know 2° Carbocations More Stable Than 1° • Major Product Formed From More Stable C+ Intermediate • Means TS in 2° Carbocation Pathway Lower in Energy • Lower Energy of Activation • Activation Energies in 1° Carbocation Pathways Much Larger
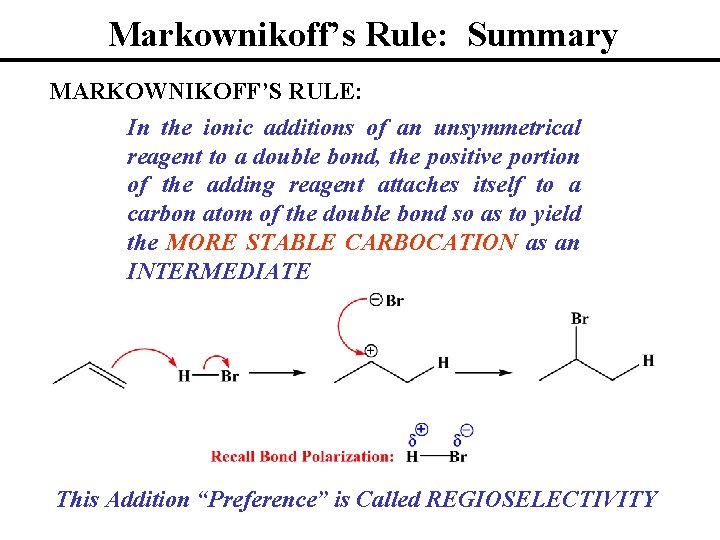
Markownikoff’s Rule: Summary MARKOWNIKOFF’S RULE: In the ionic additions of an unsymmetrical reagent to a double bond, the positive portion of the adding reagent attaches itself to a carbon atom of the double bond so as to yield the MORE STABLE CARBOCATION as an INTERMEDIATE This Addition “Preference” is Called REGIOSELECTIVITY
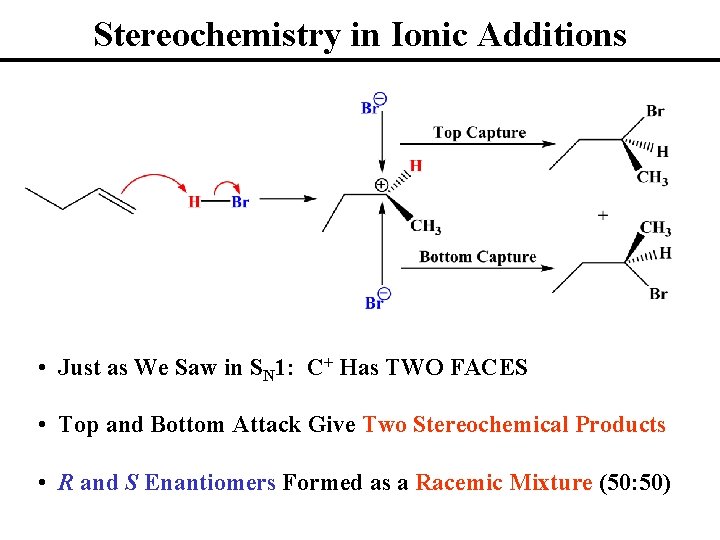
Stereochemistry in Ionic Additions • Just as We Saw in SN 1: C+ Has TWO FACES • Top and Bottom Attack Give Two Stereochemical Products • R and S Enantiomers Formed as a Racemic Mixture (50: 50)
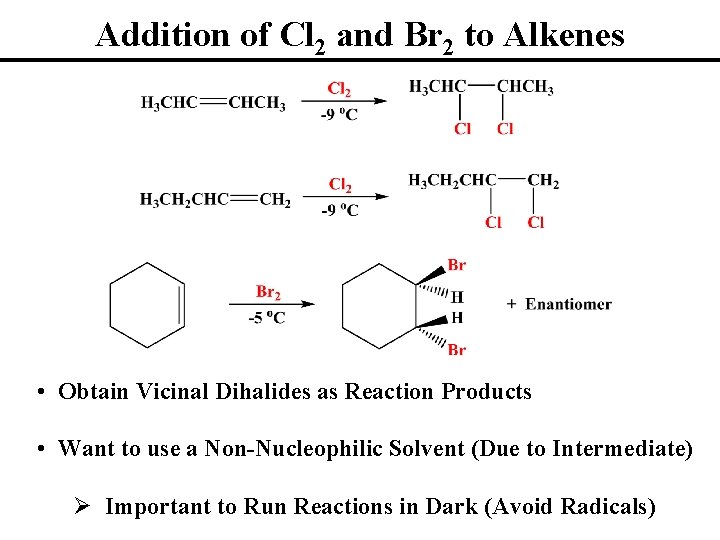
Addition of Cl 2 and Br 2 to Alkenes • Obtain Vicinal Dihalides as Reaction Products • Want to use a Non-Nucleophilic Solvent (Due to Intermediate) Ø Important to Run Reactions in Dark (Avoid Radicals)
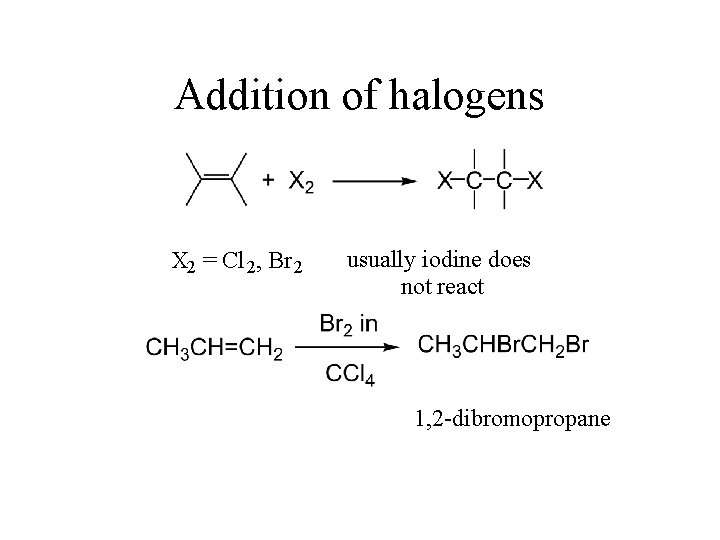
Addition of halogens X 2 = Cl 2 , Br 2 usually iodine does not react 1, 2 -dibromopropane
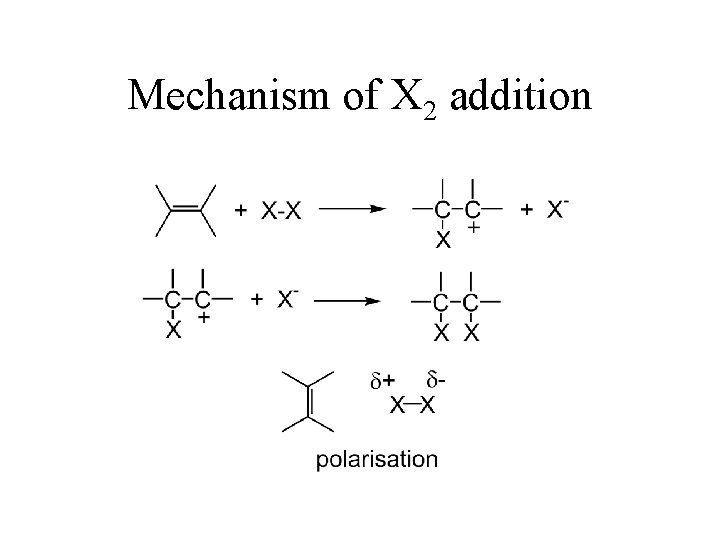
Mechanism of X 2 addition
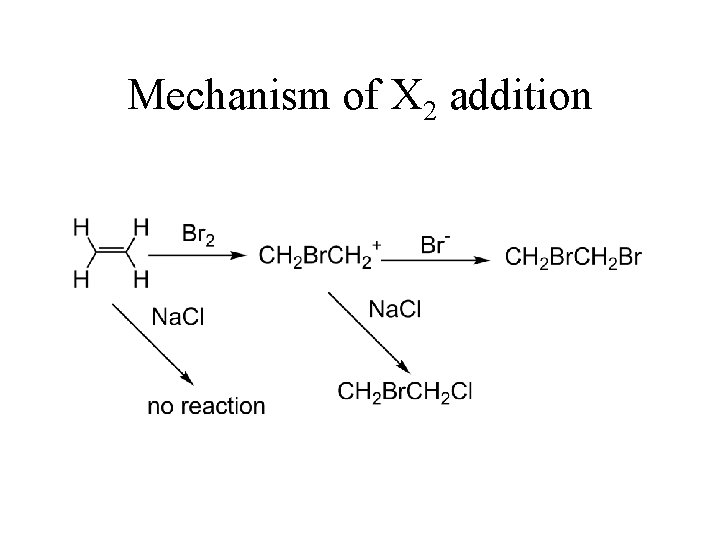
Mechanism of X 2 addition
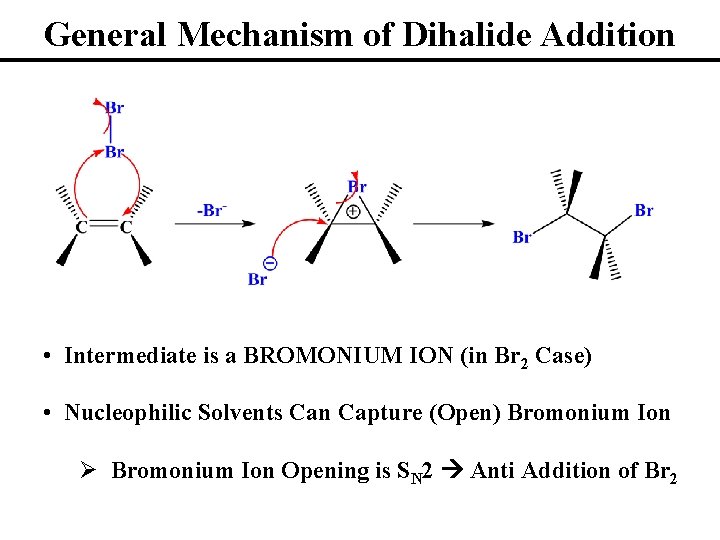
General Mechanism of Dihalide Addition • Intermediate is a BROMONIUM ION (in Br 2 Case) • Nucleophilic Solvents Can Capture (Open) Bromonium Ion Ø Bromonium Ion Opening is SN 2 Anti Addition of Br 2

Stereochemistry of Dihalide Additions • Can Open Symmetric Bromonium Ions at Either Carbon • Always (for now) Anti (Trans) Addition of X 2 • Reaction Products Are Enantiomers • Racemic Mixtures (50: 50) in Symmetric Bromonium Ions • Will Get Excess of One Enantiomer in Asymmetric Cases • Stereospecific Reactions: One Stereoiomeric Form of the Starting Material Reacts in Such a Way to Form a Specific Stereoisomeric Form of the Product
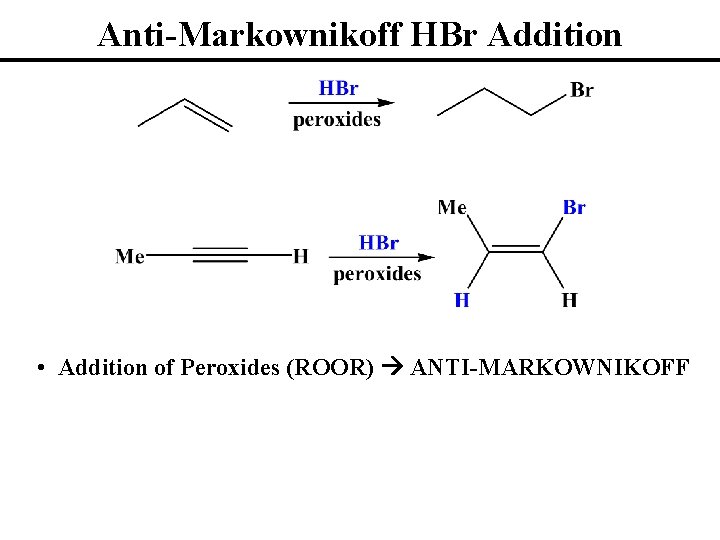
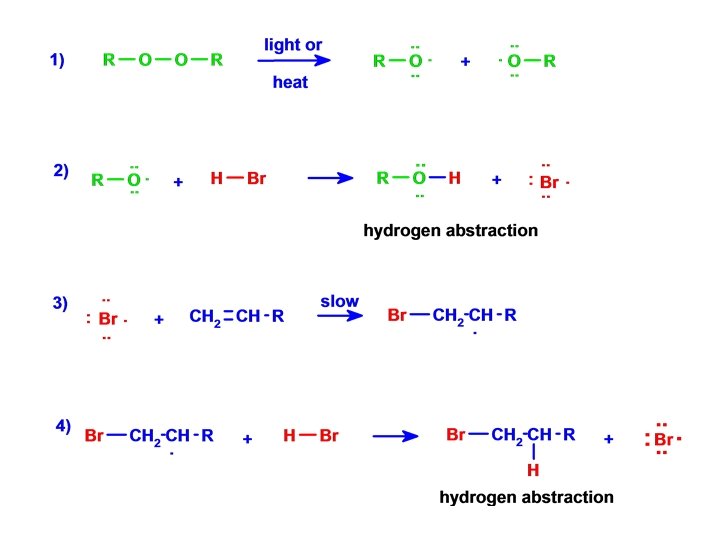
Anti-Markownikoff HBr Addition • Addition of Peroxides (ROOR) ANTI-MARKOWNIKOFF
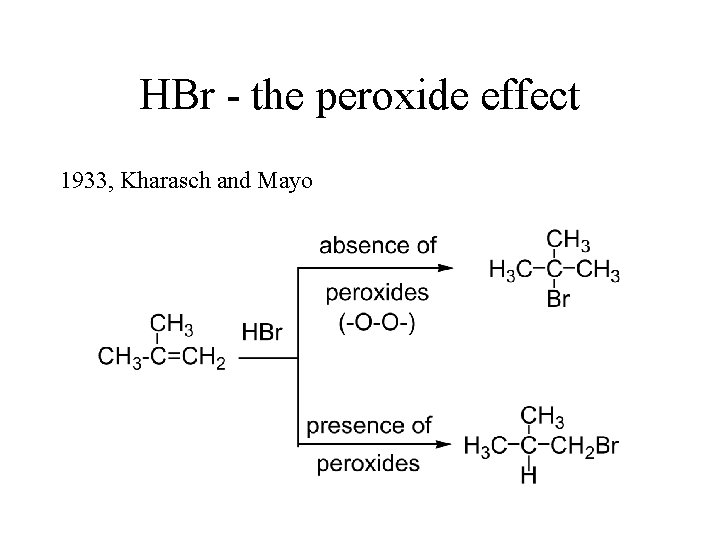
HBr - the peroxide effect 1933, Kharasch and Mayo
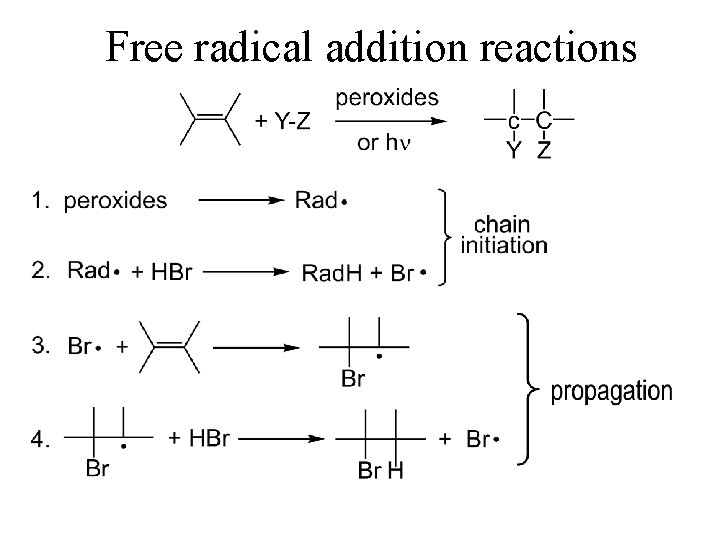
Free radical addition reactions
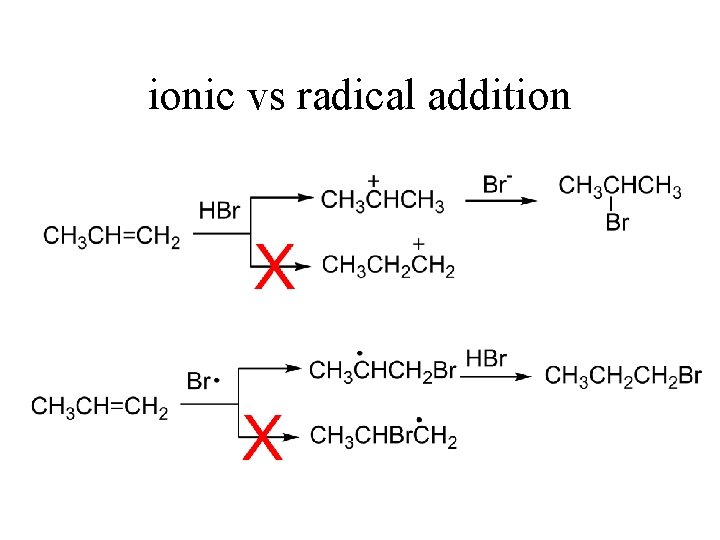
ionic vs radical addition
- Slides: 24