Avogadros Law and Ideal Gas Law Avogadros law







- Slides: 11

Avogadro’s Law and Ideal Gas Law

Avogadro’s law (also called Avogadro’s hypothesis) states: - that at the same temperature and pressure, equal volumes of different gases contain an equal number of gas particles Avogadro’s Law This means that one mole of a gas occupies the same volume as one mole of a different gas, at the same temperature and pressure. The volume of one mole of a gas is called its molar volume and is expressed in L/mol. The molar volume of any gas at STP is 22. 4 L/mol.

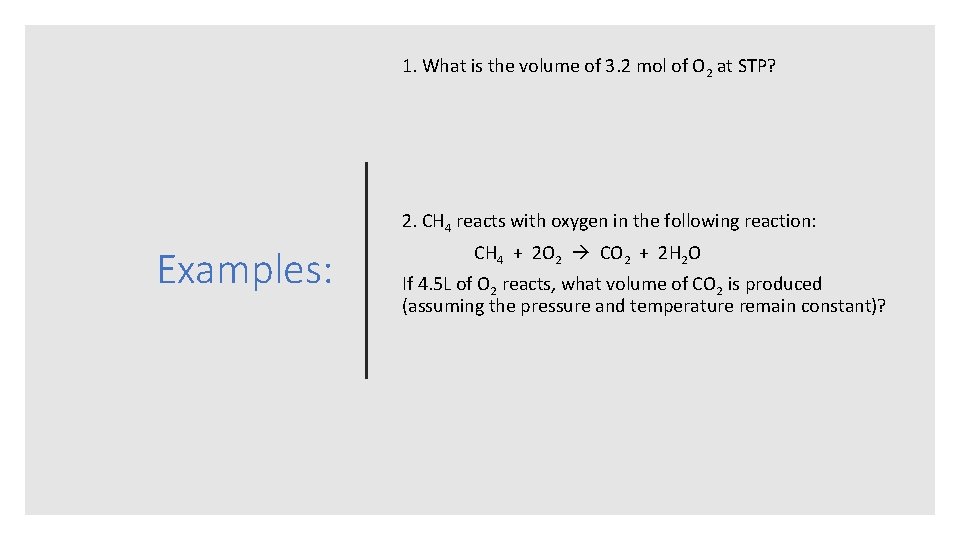
1. What is the volume of 3. 2 mol of O 2 at STP? 2. CH 4 reacts with oxygen in the following reaction: Examples: CH 4 + 2 O 2 CO 2 + 2 H 2 O If 4. 5 L of O 2 reacts, what volume of CO 2 is produced (assuming the pressure and temperature remain constant)?

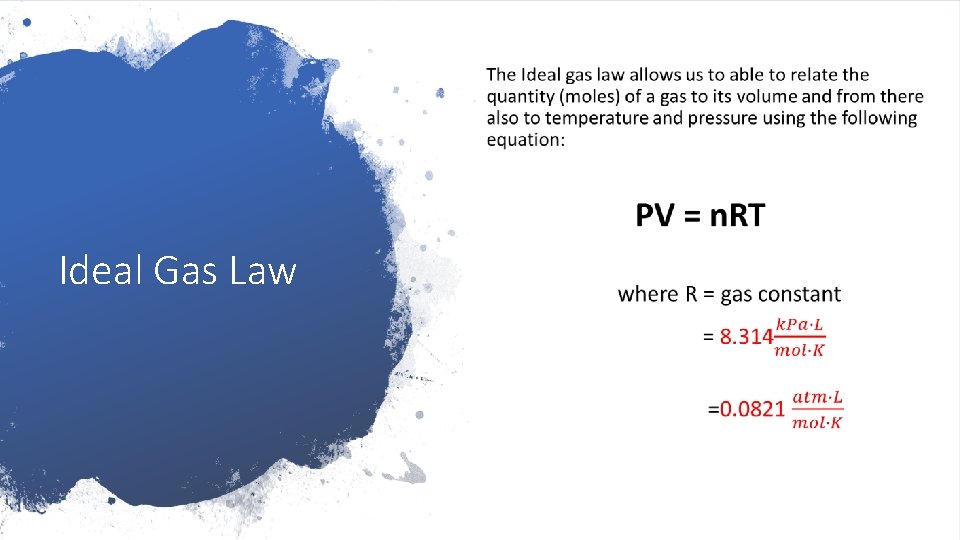
Because of Avogadro’s law, we are able to relate the quantity (moles) of a gas to its volume and from there also to temperature and pressure for ideal gases. Characteristics of “ideal gases” are: 1. composition: an ideal gas consists of a large number of identical molecules Ideal Gas Law 2. volume: the volume of the gas particles themselves is negligible to compared to the volume of the space surrounding them 3. attractive forces: the IMF between them is so weak that it is negligible 4. movement: they move in random motion

• Ideal Gas Law

Example: a) A gas cylinder with a capacity of 105 L contains helium at a pressure of 6. 70 x 103 k. Pa and a temperature of 27 o. C. Calculate the mass of helium gas in the cylinder. b) Find the density of this gas.

Example: Find the volume of 0. 48 mol of a gas at 32 o. C and 99. 6 k. Pa.

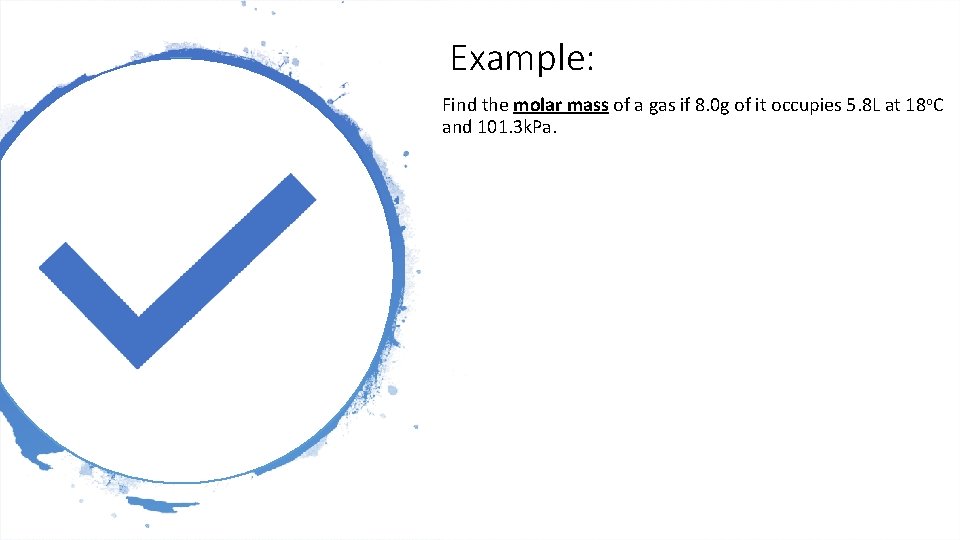
Example: Find the molar mass of a gas if 8. 0 g of it occupies 5. 8 L at 18 o. C and 101. 3 k. Pa.

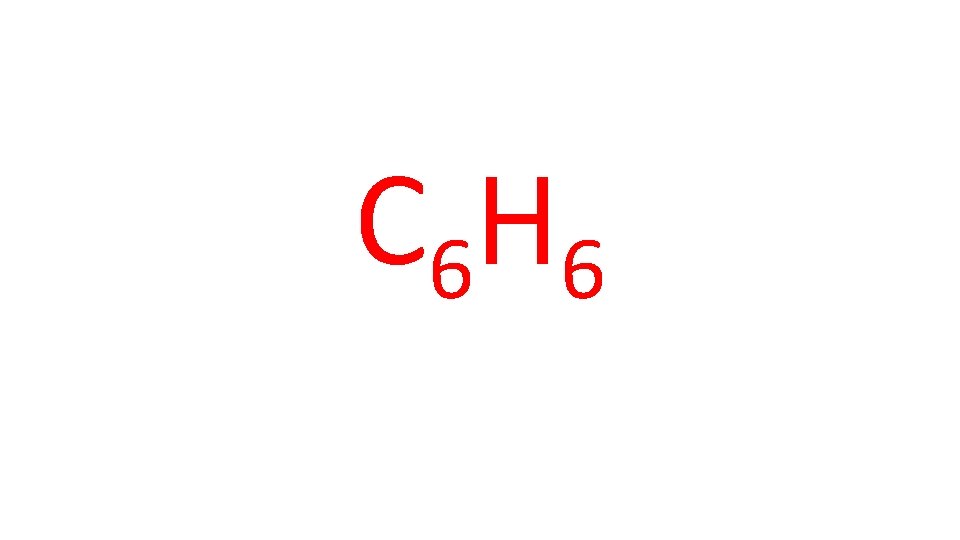
Example: Benzene consists of 92. 24% and 7. 76% of H. When a sample with a mass of 15. 62 g was placed in a container with a volume of 3. 78 L and heated to 110 o. C, the benzene vaporized and created a pressure of 168. 4 k. Pa. What is the molecular formula of benzene? hints: 1. Find moles; use moles you calculated and mass given to find the molar mass of the gas 2. Find the empirical formula of benzene 3. Use answers to 1. and 2. To find the molecular formula

C 6 H 6
