Avogadros Hypothesis The mole is a number like

Avogadro’s Hypothesis -The mole is a number like a dozen is a number or a week is a number of days (usually as measurement units) -The mole was established by using the number of atoms in 12 grams of carbon-12.
Avogadro’s Hypothesis -The number of particles in one mole is 6. 02 × 1023 (FYI, this is a very large number) 602, 350, 000, 000 -6. 02 × 1023 is also called Avogadro’s number -At standard temperature (0°C) and pressure (1 atm) (STP), 22. 4 liters of any gas will ALWAYS contain 6. 02 × 1023 particles

Avogadro’s Hypothesis (http: //www. toothpastefordinner. com/archives/2010/May/)
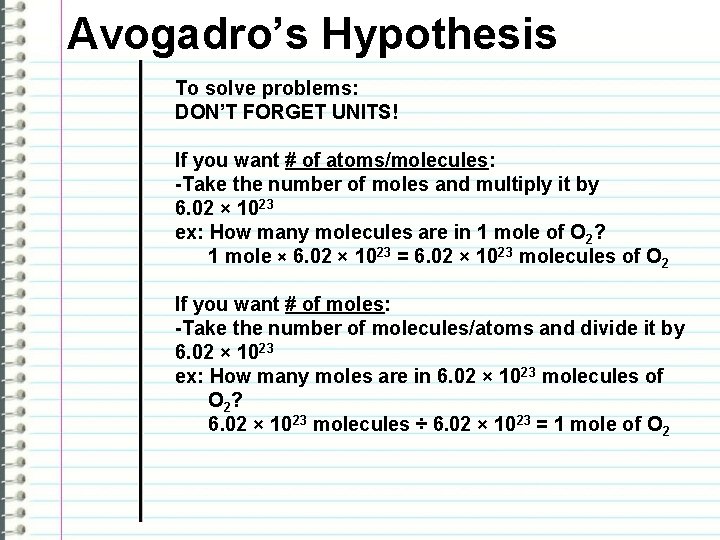
Avogadro’s Hypothesis To solve problems: DON’T FORGET UNITS! If you want # of atoms/molecules: -Take the number of moles and multiply it by 6. 02 × 1023 ex: How many molecules are in 1 mole of O 2? 1 mole × 6. 02 × 1023 = 6. 02 × 1023 molecules of O 2 If you want # of moles: -Take the number of molecules/atoms and divide it by 6. 02 × 1023 ex: How many moles are in 6. 02 × 1023 molecules of O 2? 6. 02 × 1023 molecules ÷ 6. 02 × 1023 = 1 mole of O 2

Avogadro’s # Problems Answer the following questions: 1. How many molecules are in 3. 00 moles of CO 2? 2. How many molecules are in 0. 500 moles of H 2 O? 3. How many atoms are in 1. 50 moles of He? 4. How many moles are in 12. 04 × 1023 molecules of H 2? 5. How many moles are in 6. 02 × 1023 atoms of Mg? 6. How many moles are in 2. 00 × 1023 particles of Na. Cl?
- Slides: 5