Avogadros Hypothesis Avogadros Hypothesis If two containers are


- Slides: 9

Avogadro’s Hypothesis

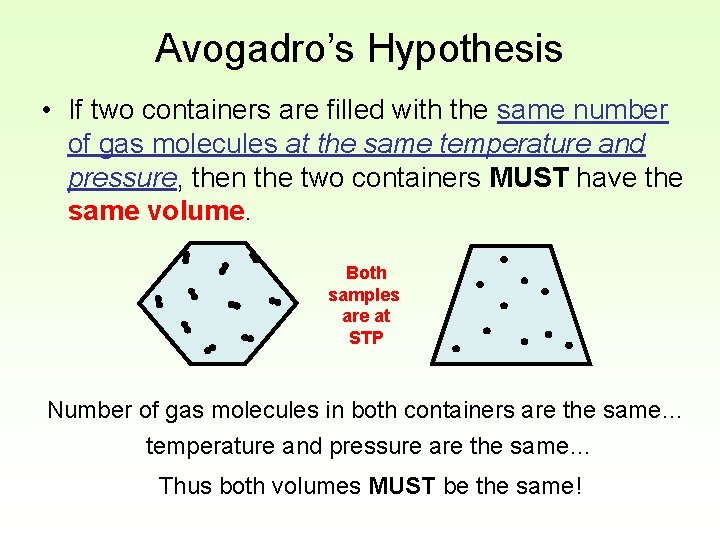
Avogadro’s Hypothesis • If two containers are filled with the same number of gas molecules at the same temperature and pressure, then the two containers MUST have the same volume. Both samples are at STP Number of gas molecules in both containers are the same… temperature and pressure are the same… Thus both volumes MUST be the same!

Avogadro’s Hypothesis • If container is filled with the half the number of gas molecules as a second container at the same temperature and pressure, then the first container must have the half the volume as the second container. Both samples are at STP Half the number of gas molecules on the left… temperature and pressure are the same… The volume on the left MUST be half!

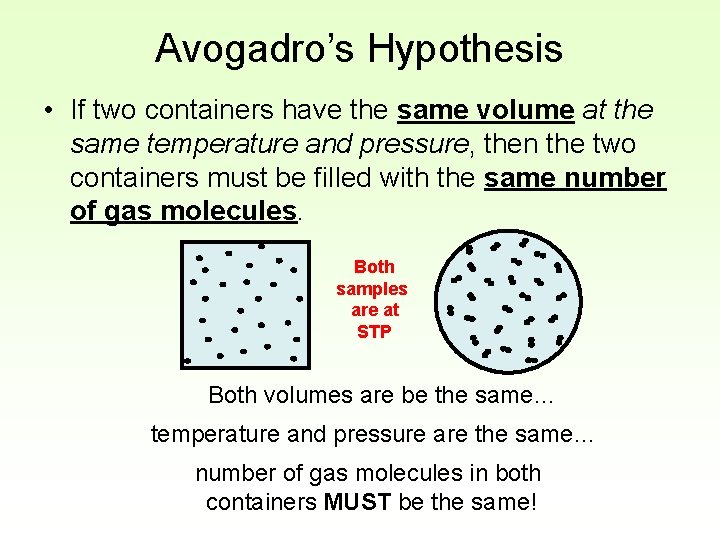
Avogadro’s Hypothesis • If two containers have the same volume at the same temperature and pressure, then the two containers must be filled with the same number of gas molecules. Both samples are at STP Both volumes are be the same… temperature and pressure are the same… number of gas molecules in both containers MUST be the same!

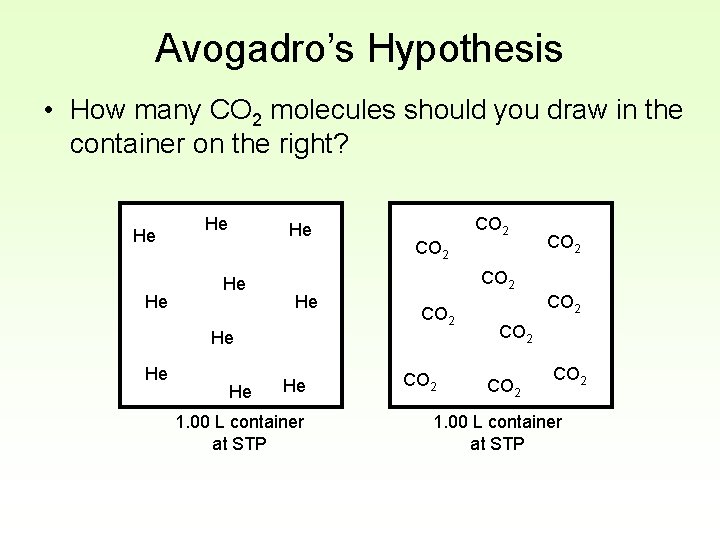
Avogadro’s Hypothesis • How many CO 2 molecules should you draw in the container on the right? He He He CO 2 He He He CO 2 He 1. 00 L container at STP CO 2 CO 2 1. 00 L container at STP

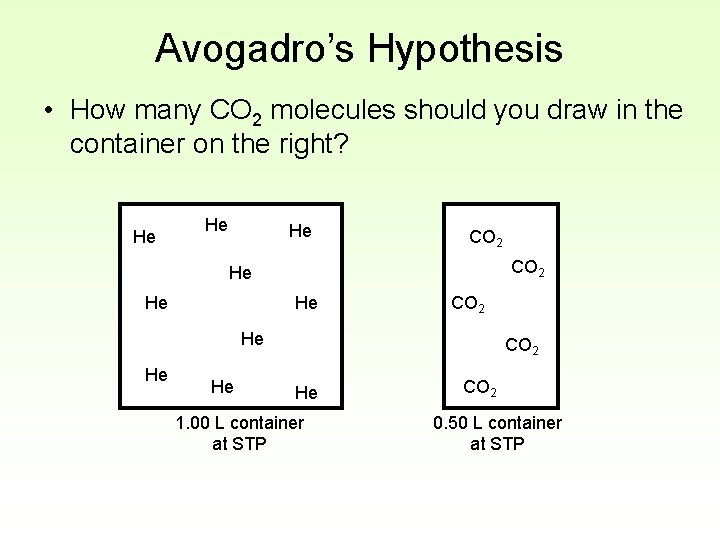
Avogadro’s Hypothesis • How many CO 2 molecules should you draw in the container on the right? He He He CO 2 He 1. 00 L container at STP CO 2 0. 50 L container at STP

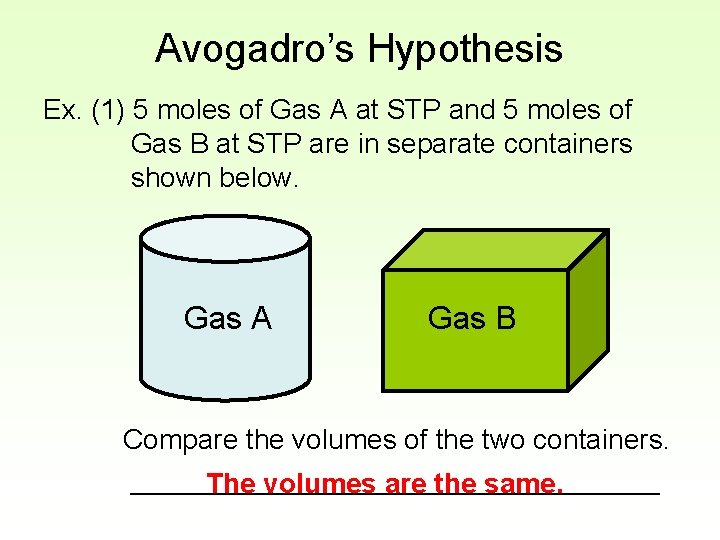
Avogadro’s Hypothesis Ex. (1) 5 moles of Gas A at STP and 5 moles of Gas B at STP are in separate containers shown below. Gas A Gas B Compare the volumes of the two containers. _________________ The volumes are the same.

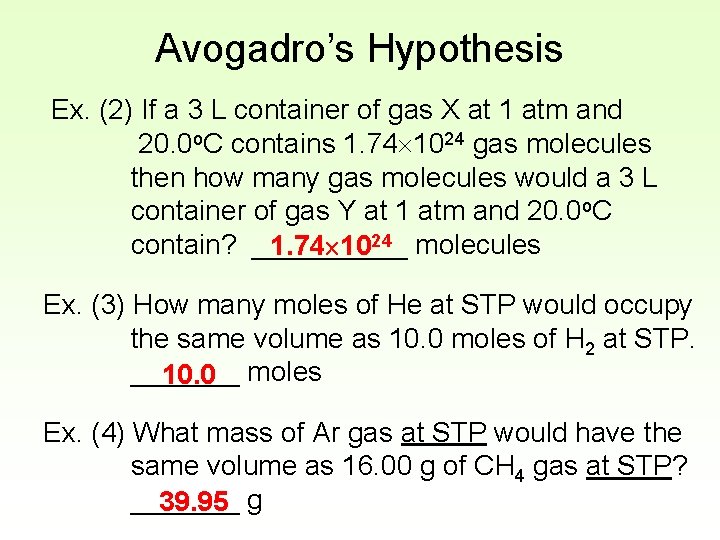
Avogadro’s Hypothesis Ex. (2) If a 3 L container of gas X at 1 atm and 20. 0 o. C contains 1. 74 1024 gas molecules then how many gas molecules would a 3 L container of gas Y at 1 atm and 20. 0 o. C contain? _____ 1. 74 1024 molecules Ex. (3) How many moles of He at STP would occupy the same volume as 10. 0 moles of H 2 at STP. _______ 10. 0 moles Ex. (4) What mass of Ar gas at STP would have the same volume as 16. 00 g of CH 4 gas at STP? _______ 39. 95 g

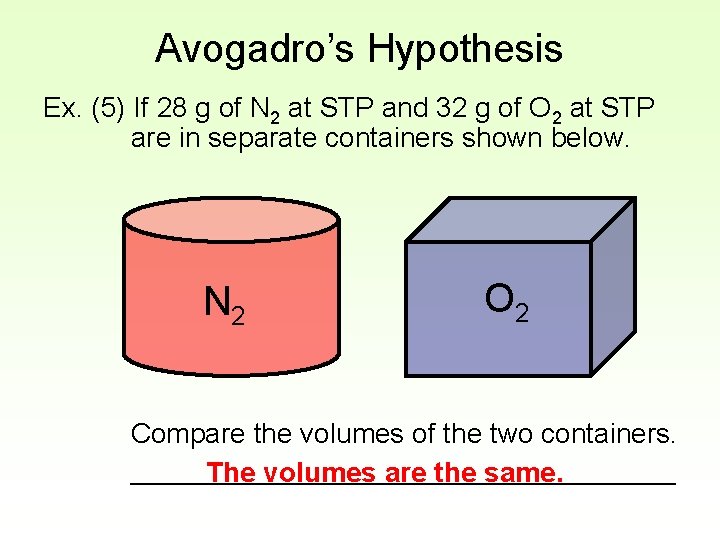
Avogadro’s Hypothesis Ex. (5) If 28 g of N 2 at STP and 32 g of O 2 at STP are in separate containers shown below. N 2 O 2 Compare the volumes of the two containers. __________________ The volumes are the same.