Average Atomic Mass How much does an atom












- Slides: 17

Average Atomic Mass

How much does an atom weigh? Ø Protons & Neutrons: 1. 67 X 10 -24 gram Ø Electrons: Ø To 9. 10 X 10 -28 gram avoid working with these really small numbers, scientists have developed the atomic mass scale.

What is an a. m. u. ? Ø atomic Ø amu mass unit 1/12 the mass of the C-12 atom. Ø C-12 is used as the reference for atomic masses. This is the STANDARD.

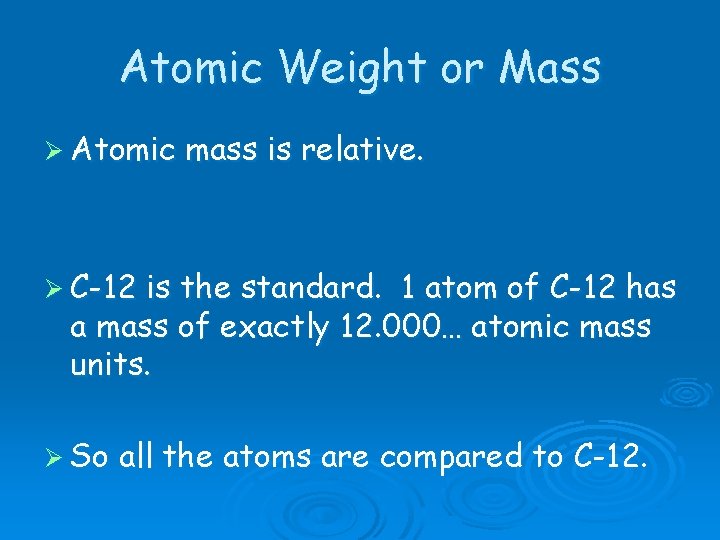
Atomic Weight or Mass Ø Atomic mass is relative. Ø C-12 is the standard. 1 atom of C-12 has a mass of exactly 12. 000… atomic mass units. Ø So all the atoms are compared to C-12.

Average Atomic Mass Ø The atomic masses reported in the periodic table represent the weighted average of the masses of the naturally occurring isotopes of that element.

Weighted Avg. of Test Grades Ø Suppose you have 1 test of 60% and 14 tests of 90%. What if I said your avg. was 75? Ø 60 + 90 = 150. Divide by 2 = 75. Ø Would you be happy?

NO! Ø The long way to do this problem: Ø (90 + 90 + 90 + 90 + 60) 15 Ø Avg. = 88. You like this a lot better!

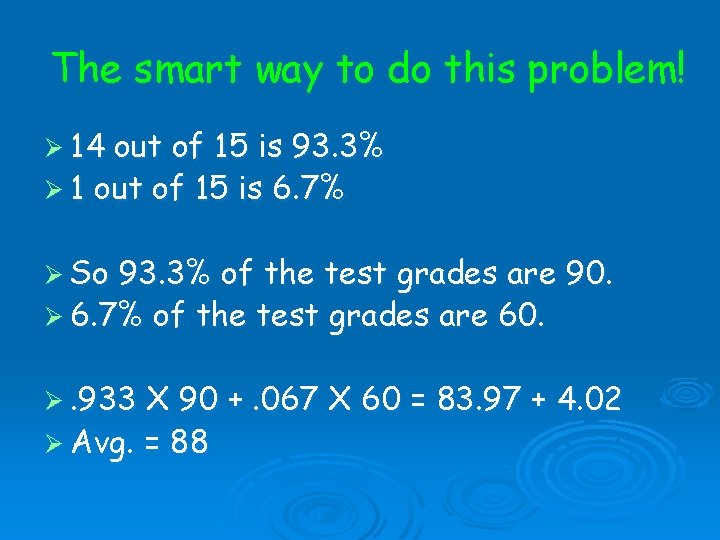
The smart way to do this problem! Ø 14 out of 15 is 93. 3% Ø 1 out of 15 is 6. 7% Ø So 93. 3% of the test grades are 90. Ø 6. 7% of the test grades are 60. Ø. 933 X 90 +. 067 X 60 = 83. 97 + 4. 02 Ø Avg. = 88

This is an even smarter way to calculate the average if you have lots of items to average.

Method of Weighted Averages 1. Convert % to decimal format. (Divide by 100%. ) 2. Multiply each isotope’s abundance factor by its atomic mass. 3. Sum.

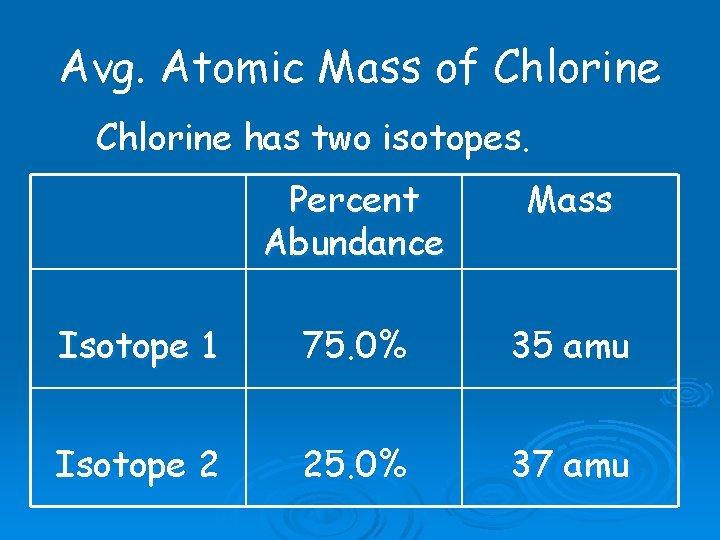
Avg. Atomic Mass of Chlorine has two isotopes. Percent Abundance Mass Isotope 1 75. 0% 35 amu Isotope 2 25. 0% 37 amu

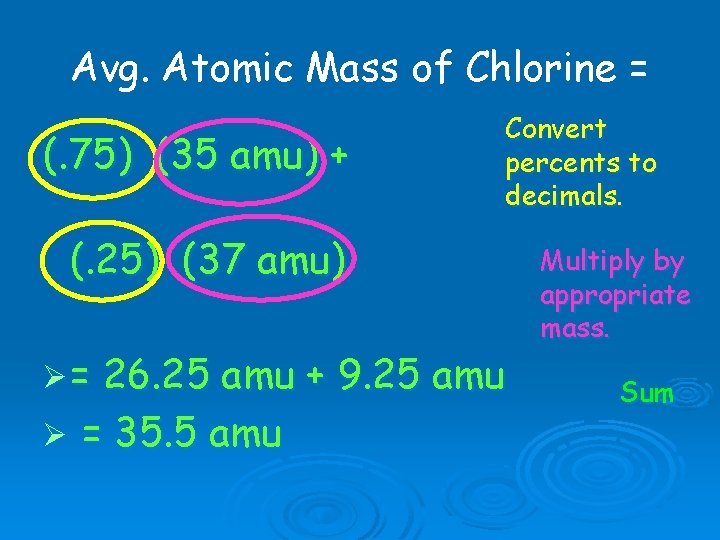
Avg. Atomic Mass of Chlorine = (. 75) (35 amu) + Convert percents to decimals. (. 25) (37 amu) Ø= 26. 25 amu + 9. 25 amu Ø = 35. 5 amu Multiply by appropriate mass. Sum

Avg. Atomic Mass of Chlorine Ø To estimate the answer: Ø 75% Cl is 35 and 25% Cl is 37. Ø The final answer has to be between 35 and 37, but closer to 35. Ø 35. 5 amu

Avg. Atomic Mass of Si 92. 21% of Si has a mass of 28 4. 70% of Si has a mass of 29 3. 09% of Si has a mass of 30

Avg. Atomic Mass of Si. 9221 X 28 25. 8188. 0470 X 29 1. 363 +. 0309 X 30 0. 927 28. 1088

Check your work! Ø Answer has to be between high & low. Ø Answer has to be closest to 28. Ø Answer is 28. 11.

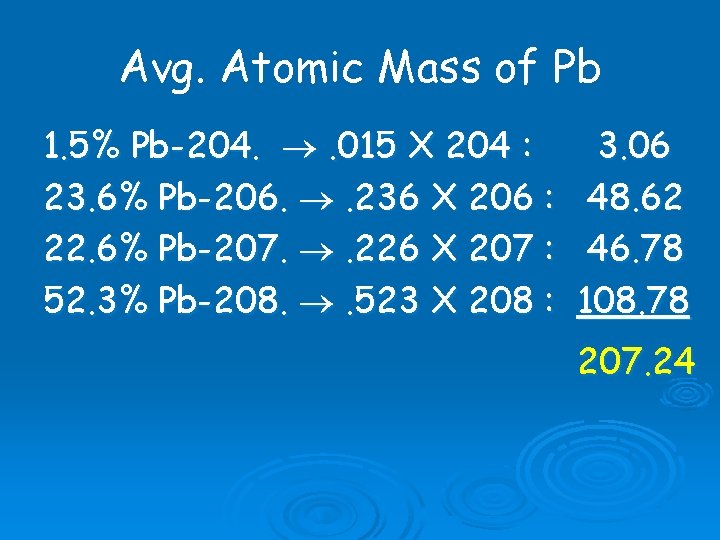
Avg. Atomic Mass of Pb 1. 5% Pb-204. . 015 X 204 : 3. 06 23. 6% Pb-206. . 236 X 206 : 48. 62 22. 6% Pb-207. . 226 X 207 : 46. 78 52. 3% Pb-208. . 523 X 208 : 108. 78 207. 24