Autosomal Recessive Polycystic Kidney Disease ARPKD The disease





























- Slides: 33

Autosomal Recessive Polycystic Kidney Disease (ARPKD) The disease with more than one face Ihab Shaheen Consultant Paediatric Nephrologist RHSC- Glasgow

Learning objectives: • • Epidemiology Genetics Clinical feature 2 Cases Differential diagnoses Prognosis Literature To take home

Epidemiology: • Inherited disorder – progressive enlargement of renal collecting ducts varying degrees of hepatic abnormality • Rare 1: 10, 000 to 1: 40, 000 • Caucasians > other ethnic groups? • M=F

Epidemiology. . . • Estimated frequency of gene 1: 70 in non isolated population • Incidence in isolated population 1: 8000 ( Finnish) • Exact incidence is unknown: 1. Published studies vary in the cohort of cases examined 2. Some severely affected babies die perinatally without definite diagnosis

Genetics: • AR • Mutation in PKHD 1, chromosome 6 P 21 • To date all kindereds with typical features of ARPKD have demonstrated linkage to this locus • Over 350 mutations have been recognized ( 2009)

Genetics. . . • Among the largest disease genes characterized to date in human genome (470 kb) • Encodes for polyductin/fibrocystin protein • This protein is localised in primary cilia mainly in the kidney • To lesser extent in liver, pancrease and arterial wall.


Genetics. . . • Genotype-phenotype correlation from type of mutation rather than the site of mutation • Truncating mutation displays severe form • Missense mutations are more frequent in less severe form

Clinical features: • • • Flank masses HTN Urinary concentration defect Hyponatraemia Renal insufficiency Pulmonary hypoplasia HSM Oesophageal varices hypersplenism

Renal: • Very rarely to be the cause of death in NN period • Usually improving following recovery from respiratory problems • Hyponatraemia is usually transient and related to the concentration defect • Fluid restriction could sort low Na but?

Hypertension • • • Unknown mechanism 80% of affected children ACE inhibitors, Ca channel blockers and beta blockers Normal renin and aldosterone peripheral activity May be related to local RAAS activation Needs more than one agent to be controlled

Case 1. . • • • 35+3 premature Caucasian male NVD, breech Birth weight: 2. 3 kg Normal antenatal scan Both parents are under 30 No F/H of renal diseases

Case 1. . . • • • Apgar of 1, 9 @1 &5 minutes 48 hrs of IV Ab Needed initially CPAP, severe RD Ventilated with surfactant x 2 Right pneumothorax, needed chest drain

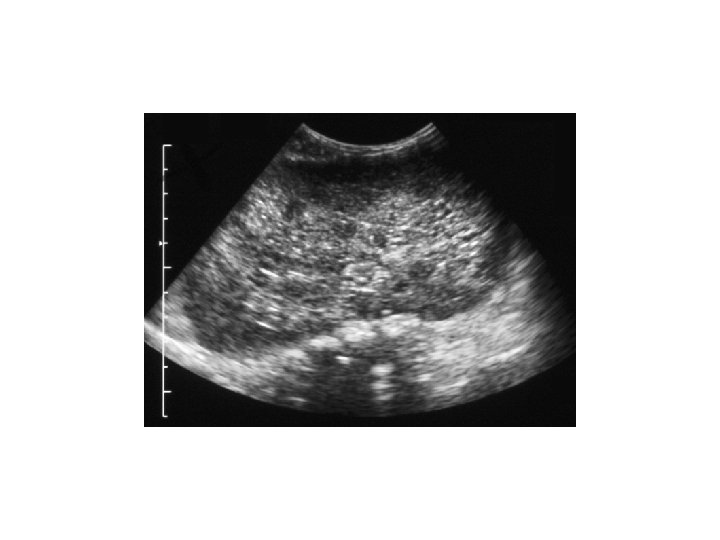
Case 1. . . • • • Generalised oedema by D 2 B/L large abdominal masses Oliguria, low Na, high urea and creatinine Urinalysis blood & protein +++ Abdominal US: B/L PKD, most likely ARPKD, coarse liver.


Case 1. . . • Issues 1. 2. 3. 4. Persistent HTN ( required 5 anti HTN& diuretics) Poor weight gain Renal failure Family counselling

Case 2. . . • • • 8 year old female Normal antenatal scan, no F/H of renal diseases 38/40, NVD RD, pneumothorax Discovered to have ARPKD at the age of 4 months Never needed dialysis

Case 2. . . • • Kept on conservative treatment Hypertension ( controlled by 2 anti HTN) No concern regarding liver Has LRD renal Tx from dad

Case 2. . . 3 months post renal Tx • Frequent blood Tx • Recurrent sepsis • Poor graft function • Massive spleenomegally

Case 2. . . Issues: 1. Graft nephrectomy 2. Haemodialysis 3. Combined liver-kidney Tx 4. Family disappointment

Diagnosis: • Diagnostic criteria were proposed by Zerres etal: 1. US features typical of ARPKD( enlarged, ecchogenic kidneys with poor CMD and 2. One or more of the following: a) absence of renal cysts in both parents particularly if they are > 30 years old

Diagnosis. . . b) clinical, lab or radiological evidence of hepatic fibrosis c) hepatic pathology demonstrating ductal plate abnormality d) previous affected sibling e) parental consanguinity suggestive of AR inheritance

Diagnosis. . . • Genetic testing is typically not required for patient with classic ARPKD • Prenatal diagnosis in a family with at least one affected child via mutation analysis • With identification and cloning of PKHD 1, molecular analysis is now available

Neonatal large kidneys • Differential diagnosis includes - ARPKD - ADPKD - Glomerulocystic disease - Diffuse cystic dysplasia • Think about syndromes, e. g. Tuberous sclerosis , Zellweger syndrome Trisomy 13 , etc…

Large NN kidneys • Other causes - RVT - Congenital NS - Contrast nephropathy - Renal candidiasis - Glycogen storage disease - Leukaemia

Clinical features of cystic diseases presenting in the newborn period Disease Inheritanc USS Pathology Associated anomalies ARPKD AR Large echogenic kidneys, microcysts, occasional macrocytes Fusiform dilatation CDs Hepatic fibrosis/ biliary dysgenesis ADPKD AD Large echogenic kidneys, occasional macrocysts (infants), multiple macrocysts (older) Cysts from any portion of nephron MV prolapse, cerebral aneurysm, AV malformation, hepatic cysts, pancreatic cysts GCKD AD/ sporadic Large echogenic kidneys, occasional macrocysts Cystic dilatations of glomeruli May be syndromic Hepatic fibrosis 10% Diffuse cystic dysplasia Sporadic Large echogenic kidneys, microcysts or macrocysts Immature nephron development, dysplastic features Usually syndromic

Clinical features suggesting ADPKD rather than ARPKD: • Positive F/H • Extrarenal cysts • Cerebral aneurysm • Asymptomatic presentation • Unilateral presentation • Haematuria • UTIs

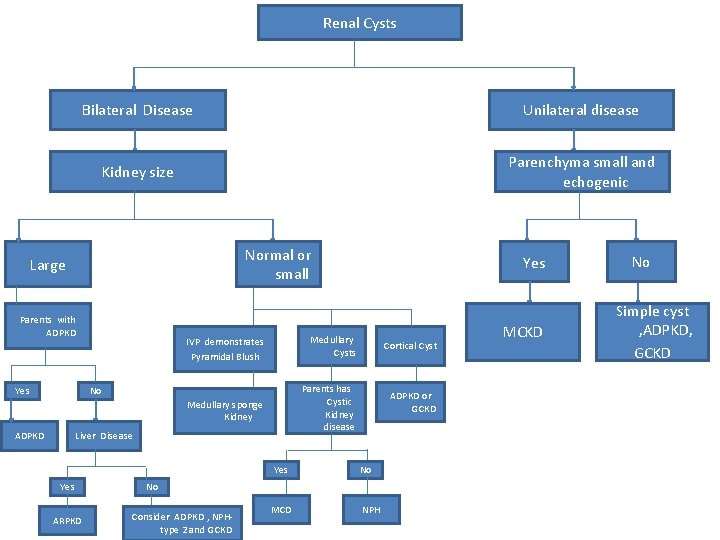
Renal Cysts Bilateral Disease Unilateral disease Kidney size Parenchyma small and echogenic Normal or small Large Parents with ADPKD Yes IVP demonstrates Pyramidal Blush Medullary Cysts Medullary sponge Kidney Parents has Cystic Kidney disease No ADPKD Liver Disease Yes ARPKD Yes Cortical Cyst ADPKD or GCKD No No Consider ADPKD , NPHtype 2 and GCKD MCD NPH MCKD No Simple cyst , ADPKD, GCKD

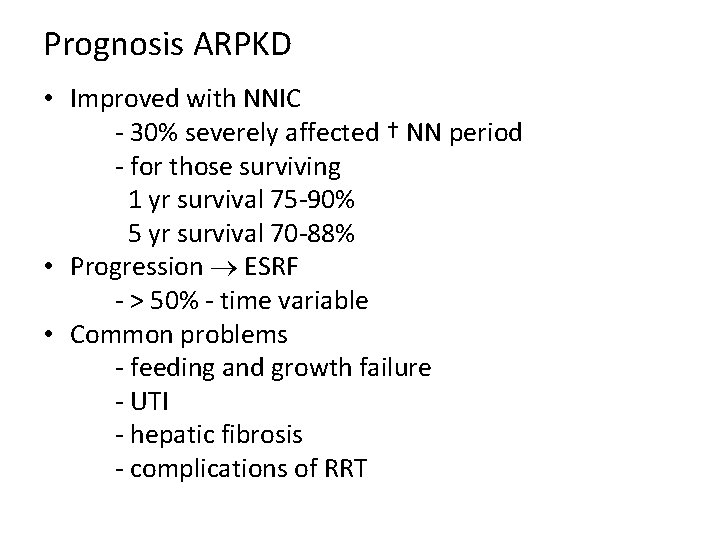
Prognosis ARPKD • Improved with NNIC - 30% severely affected † NN period - for those surviving 1 yr survival 75 -90% 5 yr survival 70 -88% • Progression ESRF - > 50% - time variable • Common problems - feeding and growth failure - UTI - hepatic fibrosis - complications of RRT

Prognosis ARPKD. . . • Portal HT not uncommon • Significant risk for ascending bacterial cholangitis • Hepatic complications are the main reason of death in a 14 year follow up study post renal Tx

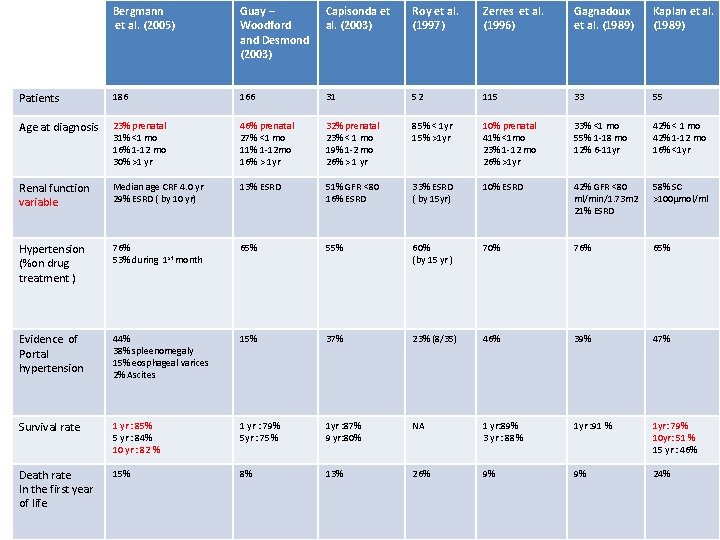
Bergmann et al. (2005) Guay – Woodford and Desmond (2003) Capisonda et al. (2003) Roy et al. (1997) Zerres et al. (1996) Gagnadoux et al. (1989) Kaplan et al. (1989) Patients 186 166 31 52 115 33 55 Age at diagnosis 23% prenatal 31% <1 mo 16% 1 -12 mo 30% >1 yr 46% prenatal 27% <1 mo 11% 1 -12 mo 16% > 1 yr 32% prenatal 23% < 1 mo 19% 1 -2 mo 26% > 1 yr 85% < 1 yr 15% >1 yr 10% prenatal 41% <1 mo 23% 1 -12 mo 26% >1 yr 33% <1 mo 55% 1 -18 mo 12% 6 -11 yr 42% < 1 mo 42% 1 -12 mo 16% <1 yr Renal function variable Median age CRF 4. 0 yr 29% ESRD ( by 10 yr) 13% ESRD 51% GFR <80 16% ESRD 33% ESRD ( by 15 yr) 10% ESRD 42% GFR <80 ml/min/1. 73 m 2 21% ESRD 58% SC >100µmol/ml Hypertension (%on drug treatment ) 76% 53% during 1 st month 65% 55% 60% (by 15 yr ) 70% 76% 65% Evidence of Portal hypertension 44% 38% spleenomegaly 15% eosphageal varices 2% Ascites 15% 37% 23% (8/35) 46% 39% 47% Survival rate 1 yr : 85% 5 yr : 84% 10 yr : 82 % 1 yr : 79% 5 yr : 75% 1 yr : 87% 9 yr: 80% NA 1 yr: 89% 3 yr : 88% 1 yr : 91 % 1 yr: 79% 10 yr: 51 % 15 yr : 46% Death rate In the first year of life 15% 8% 13% 26% 9% 9% 24%

To take home • Significant phenotypic variability • Neonatal period - respiratory - fluid balance and electrolytes - hypertension • Prognosis improved with NNIC but progression to ESRF in >50%

To take home • Portal HTN can develop with normal synthetic liver function • Every patient with recurrent unexplained sepsis should be treated for cholangitis ( gram neg organisms) • Dietetic input is crucial • BP control could be a challenge