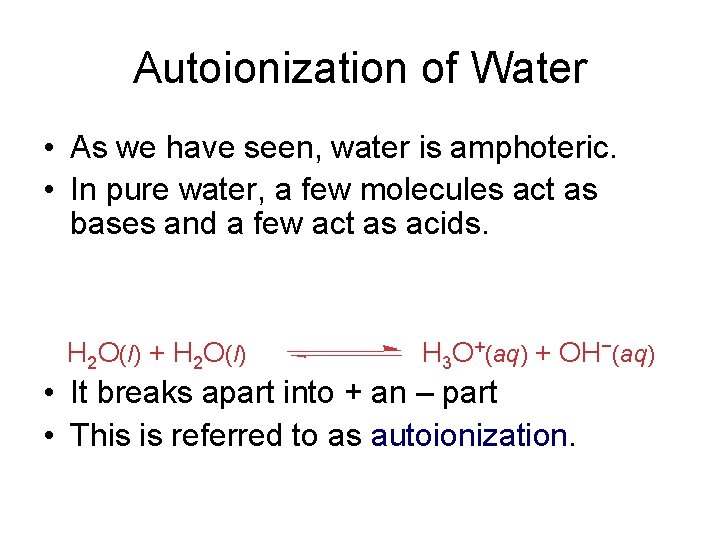
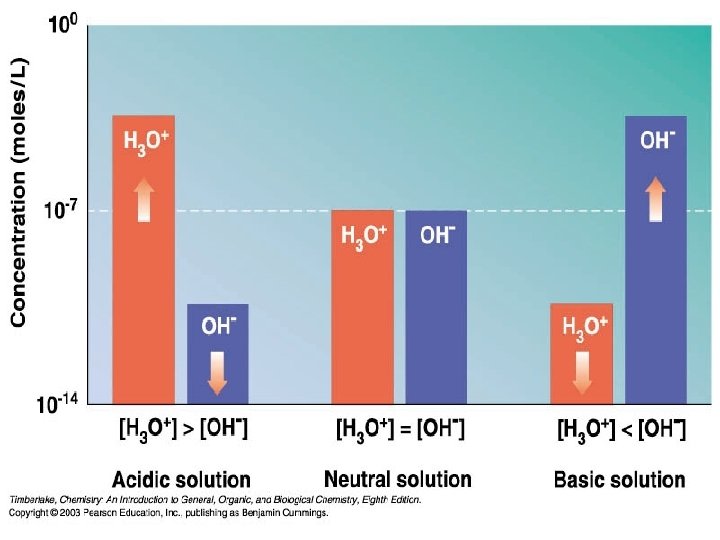
Autoionization of Water As we have seen water
![I. Calculating p. H A. p. H= -log [H+] or –log [H 3 O+] I. Calculating p. H A. p. H= -log [H+] or –log [H 3 O+]](https://slidetodoc.com/presentation_image/d2f07cd52af66616d7bf00e8f77fcd09/image-3.jpg)
![Calculating the p. H = - log [H+] (Remember that the [ ] mean Calculating the p. H = - log [H+] (Remember that the [ ] mean](https://slidetodoc.com/presentation_image/d2f07cd52af66616d7bf00e8f77fcd09/image-9.jpg)

![The p. H of a Solution • p. H = log[H 3 O+] and The p. H of a Solution • p. H = log[H 3 O+] and](https://slidetodoc.com/presentation_image/d2f07cd52af66616d7bf00e8f77fcd09/image-13.jpg)
- Slides: 13


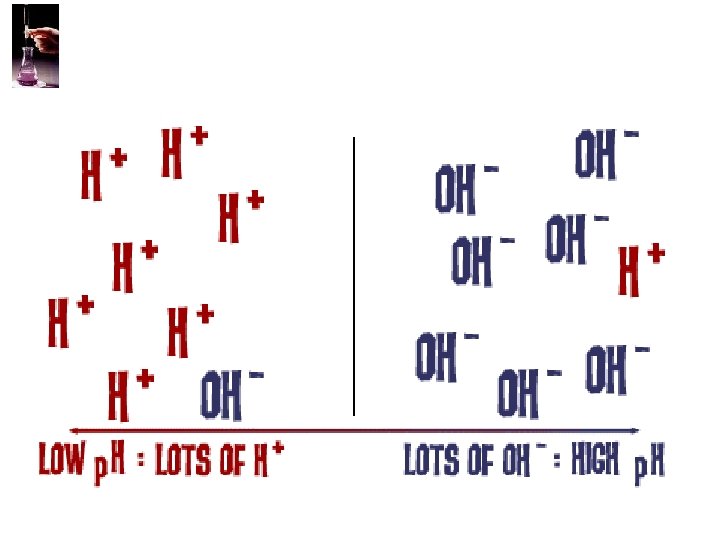
Autoionization of Water • As we have seen, water is amphoteric. • In pure water, a few molecules act as bases and a few act as acids. H 2 O(l) + H 2 O(l) H 3 O+(aq) + OH−(aq) • It breaks apart into + an – part • This is referred to as autoionization.
![I Calculating p H A p H log H or log H 3 O I. Calculating p. H A. p. H= -log [H+] or –log [H 3 O+]](https://slidetodoc.com/presentation_image/d2f07cd52af66616d7bf00e8f77fcd09/image-3.jpg)
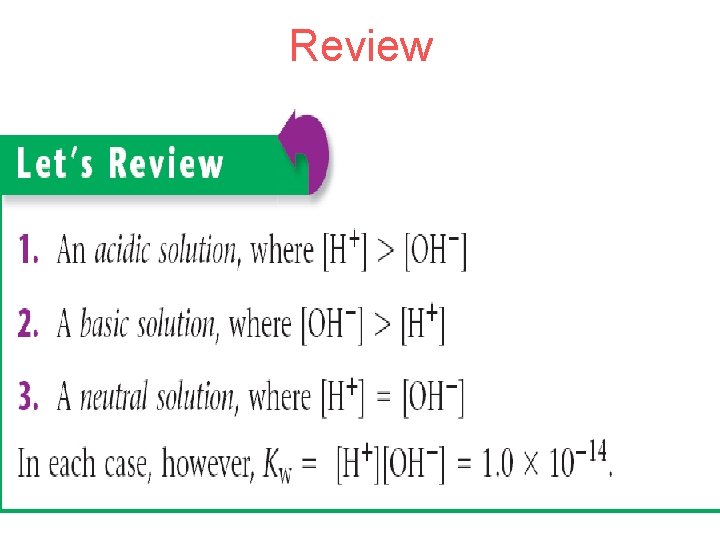
I. Calculating p. H A. p. H= -log [H+] or –log [H 3 O+] B. p. OH= -log [OH-] C. p. H + p. OH = 14 Neutral p. H 0 Acidic p. OH 14 7 Basic 14 0

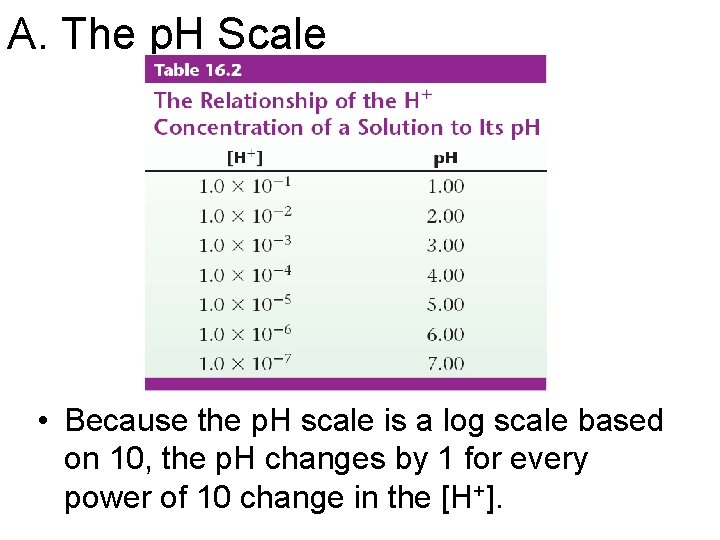
A. The p. H Scale • Because the p. H scale is a log scale based on 10, the p. H changes by 1 for every power of 10 change in the [H+].



Review

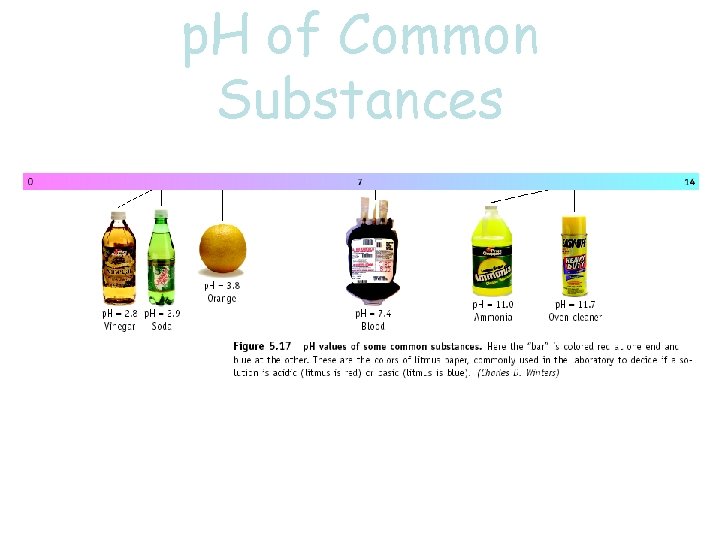
p. H of Common Substances
![Calculating the p H log H Remember that the mean Calculating the p. H = - log [H+] (Remember that the [ ] mean](https://slidetodoc.com/presentation_image/d2f07cd52af66616d7bf00e8f77fcd09/image-9.jpg)
Calculating the p. H = - log [H+] (Remember that the [ ] mean Molarity) Example: If [H+] = 1 X 10 -10 p. H = - log 1 X 10 -10 p. H = - (- 10) p. H = 10 Example: If [H+] = 1. 8 X 10 -5 p. H = - log 1. 8 X 10 -5 p. H = - (- 4. 74) p. H = 4. 74

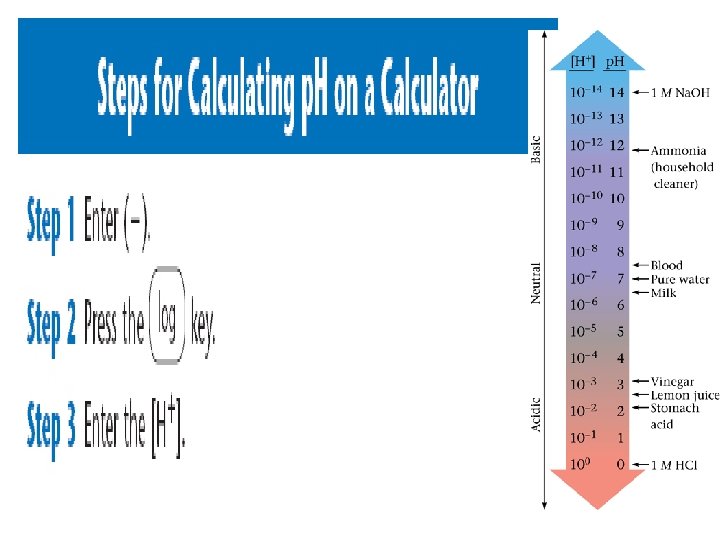
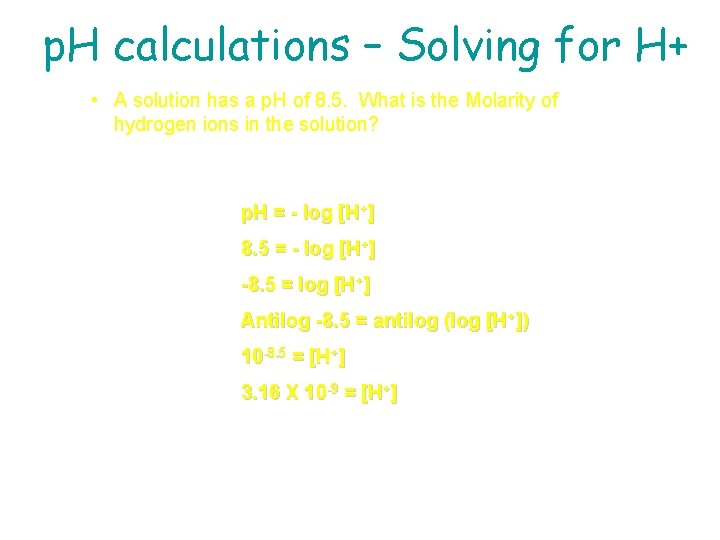
p. H calculations – Solving for H+ If the p. H of Coke is 3. 12, [H+] = ? ? ? Because p. H = - log [H+] then - p. H = log [H+] Take antilog (10 x) of both sides and get 10 -p. H = [H+] = 10 -3. 12 = 7. 6 x 10 -4 M *** to find antilog on your calculator, look for “Shift” or “ 2 nd function” and then the log button

p. H calculations – Solving for H+ • A solution has a p. H of 8. 5. What is the Molarity of hydrogen ions in the solution? p. H = - log [H+] 8. 5 = - log [H+] -8. 5 = log [H+] Antilog -8. 5 = antilog (log [H+]) 10 -8. 5 = [H+] 3. 16 X 10 -9 = [H+]

![The p H of a Solution p H logH 3 O and The p. H of a Solution • p. H = log[H 3 O+] and](https://slidetodoc.com/presentation_image/d2f07cd52af66616d7bf00e8f77fcd09/image-13.jpg)
The p. H of a Solution • p. H = log[H 3 O+] and [H 3 O+] = 10 p. H – Acidic p. H < 7. 00 – Neutral p. H = 7. 00 – Basic p. H > 7. 00 E. g. determine the p. H of a solution in which [H 3 O+] = 5. 40 x 10 6 M E. g. 2 determine the p. H of a solution in which the [OH ] = 3. 33 x 10 3 M E. g. 3 determine the p. OH of a solution in which the [OH ] = 3. 33 x 10 3 M E. g. 4 Determine the [H 3 O+] if the p. H of the solution is 7. 35. • The term p. X is defined in exactly the same way as p. H. Eg. 5 What is the p. Ca if [Ca 2+] = 6. 44 x 10 -4