Atrial Shunt for HFp EF New Devices and






- Slides: 17

Atrial Shunt for HFp. EF: New Devices and Clinical Program Update William T. Abraham, MD, FACP, FACC, FAHA, FESC, FRCP Professor of Medicine, Physiology, and Cell Biology College of Medicine Distinguished Professor The Ohio State University Columbus, Ohio

William T Abraham, MD Employee, Stockholder: V-Wave Medical

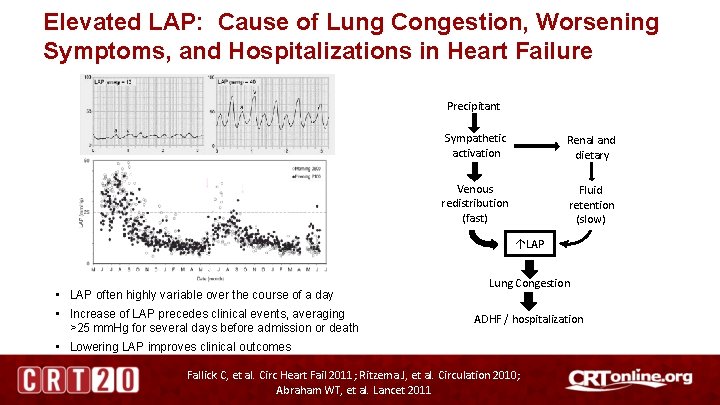
Elevated LAP: Cause of Lung Congestion, Worsening Symptoms, and Hospitalizations in Heart Failure Precipitant Sympathetic activation Renal and dietary Venous redistribution (fast) Fluid retention (slow) ↑LAP • LAP often highly variable over the course of a day • Increase of LAP precedes clinical events, averaging >25 mm. Hg for several days before admission or death Lung Congestion ADHF / hospitalization • Lowering LAP improves clinical outcomes Fallick C, et al. Circ Heart Fail 2011; Ritzema J, et al. Circulation 2010; Abraham WT, et al. Lancet 2011

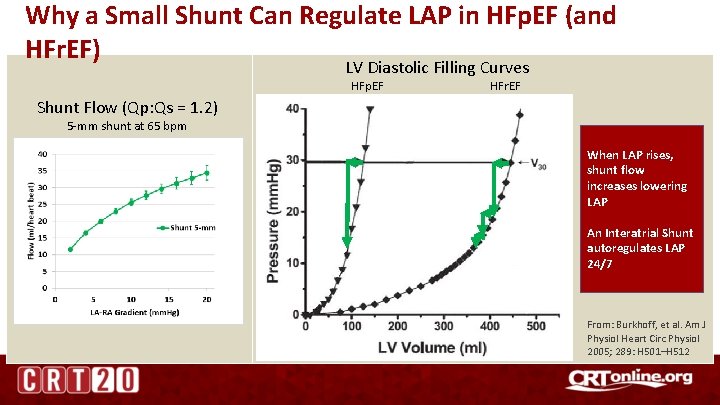
Why a Small Shunt Can Regulate LAP in HFp. EF (and HFr. EF) LV Diastolic Filling Curves HFp. EF HFr. EF Shunt Flow (Qp: Qs = 1. 2) 5 -mm shunt at 65 bpm When LAP rises, shunt flow increases lowering LAP An Interatrial Shunt autoregulates LAP 24/7 From: Burkhoff, et al. Am J Physiol Heart Circ Physiol 2005; 289: H 501–H 512

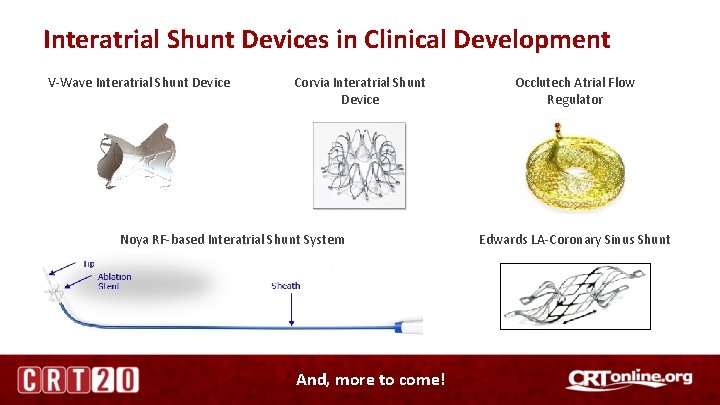
Interatrial Shunt Devices in Clinical Development V-Wave Interatrial Shunt Device Corvia Interatrial Shunt Device Noya RF-based Interatrial Shunt System And, more to come! Occlutech Atrial Flow Regulator Edwards LA-Coronary Sinus Shunt

Evidence Supporting Inter-Atrial Shunt Therapy in Chronic Heart Failure • Patients with mitral valve stenosis and an atrial septal defect (ASD) have fewer symptoms than patients with an intact septum 1 • Closure of ASDs in patients with unrecognized left ventricular dysfunction results in elevated LAP and pulmonary edema 2 • Pre-clinical animal studies demonstrate hemodynamic, echocardiographic, and survival benefits with interatrial shunting 3 • First-in-human and clinical pilot studies support the safety, feasibility, and potential effectiveness of interatrial shunting in heart failure 4 -10 1. Lutembacher R. Arch Mal Coeur 1916 2. Ewert P, et al. Catheter Cardiovasc Interv 2001 3. Eigler N, et al. Structural Heart 2017 4. Søndergaard L, et al. Eur Heart J 2014 5. Hasenfuβ, et al. Lancet 2016 6. Feldman et al. Circulation 2017 7. Del Trigo M, et al. Lancet 2016 8. Rodés-Cabau J, et al. JACC Intv 2018 9. Paitazoglou C, et al. Euro. Interv 2019 10. Guimmarães L, et al. Euro. Interv 2020 (in press)

V-Wave Shunt Device: Preclinical Evidence Chronic Heart Failure Animal Study • Performed at The Ohio State University • HF induced by performing 2 consecutive injections of microspheres into circumflex coronary artery creating myocardial infarction (ischemic heart failure) in sheep • The model is associated with very high 12 -week mortality and continuous degradation in hemodynamic parameters from baseline • Fluid filled catheters and Echo done weekly including RAP, PAP, LAP, saturations, and LV parameters such as EF and dimensions • 14 animals implanted with shunts and 7 animals served as control • Study duration was 12 weeks

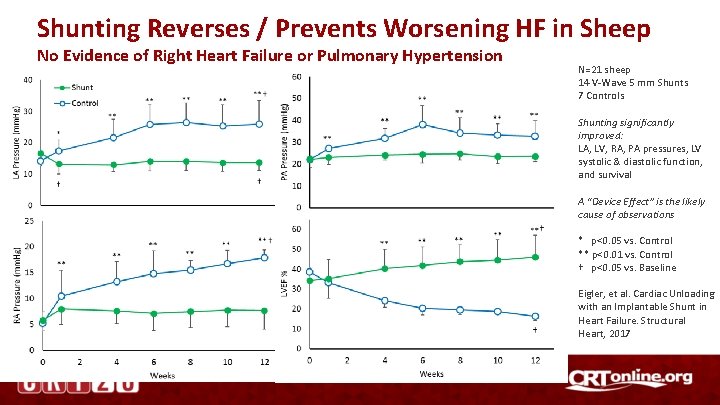
Shunting Reverses / Prevents Worsening HF in Sheep No Evidence of Right Heart Failure or Pulmonary Hypertension N=21 sheep 14 V-Wave 5 mm Shunts 7 Controls Shunting significantly improved: LA, LV, RA, PA pressures, LV systolic & diastolic function, and survival A “Device Effect” is the likely cause of observations * p<0. 05 vs. Control ** p<0. 01 vs. Control † p<0. 05 vs. Baseline Eigler, et al. Cardiac Unloading with an Implantable Shunt in Heart Failure. Structural Heart, 2017

First REDUCE LAP-HF Trial • Prospective, non-randomized study • Symptomatic HF (N=64) • Preserved EF (>40%) only • Elevated PCWP at rest (>15 mm. Hg) or during exercise (>25 mm. Hg) • Monitored by independent DSMB and CEC • Assessed by independent Core-Laboratories • Echo • Hemodynamic • Planned three year clinical follow-up Hasenfuβ, et al. Lancet 2016

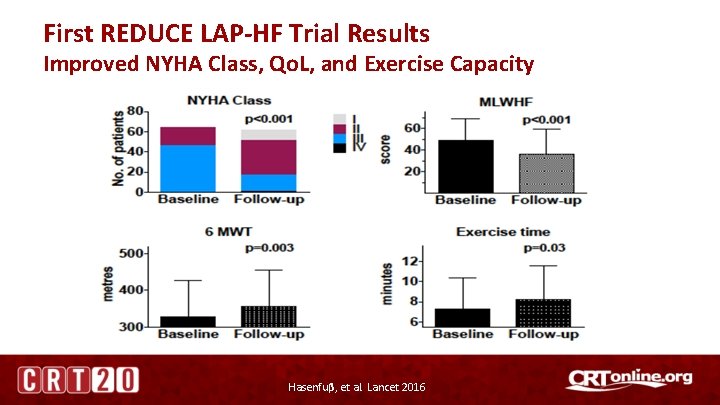
First REDUCE LAP-HF Trial Results Improved NYHA Class, Qo. L, and Exercise Capacity Hasenfuβ, et al. Lancet 2016

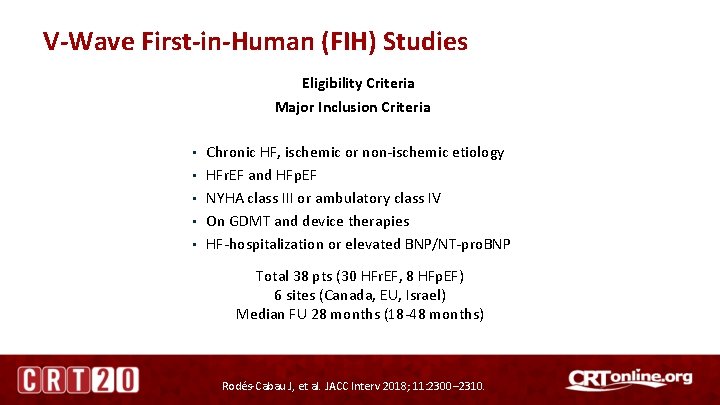
V-Wave First-in-Human (FIH) Studies Eligibility Criteria Major Inclusion Criteria • • • Chronic HF, ischemic or non-ischemic etiology HFr. EF and HFp. EF NYHA class III or ambulatory class IV On GDMT and device therapies HF-hospitalization or elevated BNP/NT-pro. BNP Total 38 pts (30 HFr. EF, 8 HFp. EF) 6 sites (Canada, EU, Israel) Median FU 28 months (18 -48 months) Rodés-Cabau J, et al. JACC Interv 2018; 11: 2300– 2310.

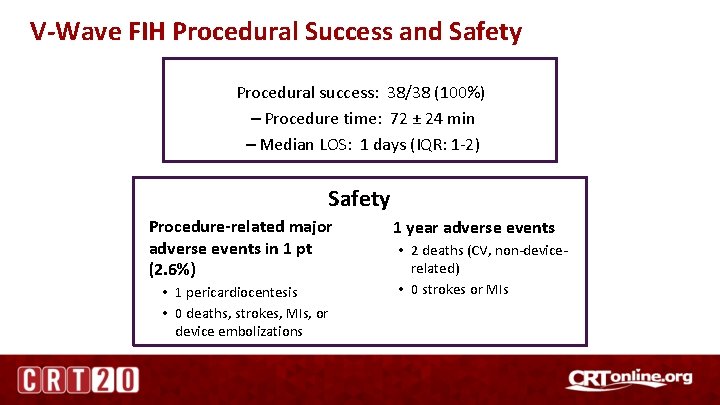
V-Wave FIH Procedural Success and Safety Procedural success: 38/38 (100%) – Procedure time: 72 ± 24 min – Median LOS: 1 days (IQR: 1 -2) Safety Procedure-related major adverse events in 1 pt (2. 6%) • 1 pericardiocentesis • 0 deaths, strokes, MIs, or device embolizations 1 year adverse events • 2 deaths (CV, non-devicerelated) • 0 strokes or MIs

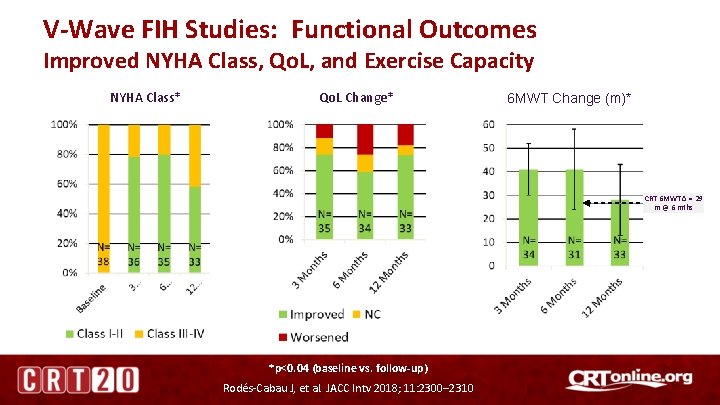
V-Wave FIH Studies: Functional Outcomes Improved NYHA Class, Qo. L, and Exercise Capacity NYHA Class* Qo. L Change* 6 MWT Change (m)* CRT 6 MWT Δ = 29 m @ 6 mths *p<0. 04 (baseline vs. follow-up) Rodés-Cabau J, et al. JACC Intv 2018; 11: 2300– 2310

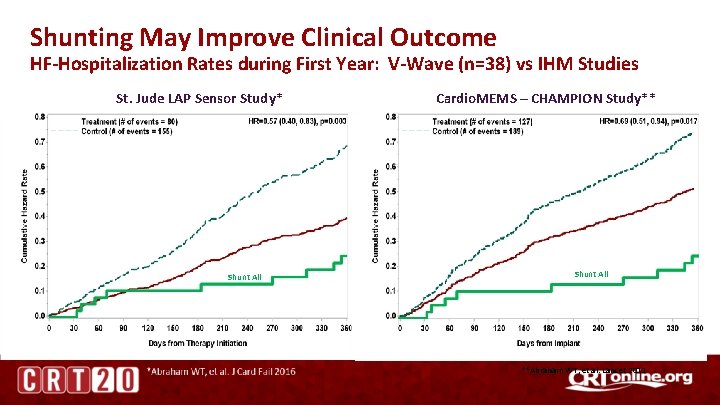
Shunting May Improve Clinical Outcome HF-Hospitalization Rates during First Year: V-Wave (n=38) vs IHM Studies St. Jude LAP Sensor Study* Shunt All Cardio. MEMS – CHAMPION Study** Shunt All **Abraham WT, et al. Lancet 2011

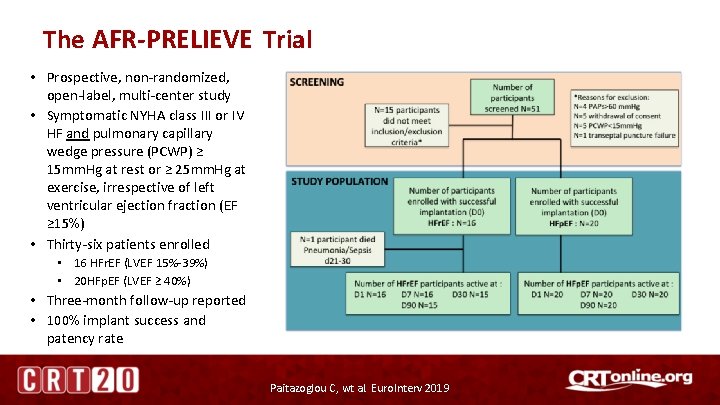
The AFR-PRELIEVE Trial • Prospective, non-randomized, open-label, multi-center study • Symptomatic NYHA class III or IV HF and pulmonary capillary wedge pressure (PCWP) ≥ 15 mm. Hg at rest or ≥ 25 mm. Hg at exercise, irrespective of left ventricular ejection fraction (EF ≥ 15%) • Thirty-six patients enrolled • 16 HFr. EF (LVEF 15%-39%) • 20 HFp. EF (LVEF ≥ 40%) • Three-month follow-up reported • 100% implant success and patency rate Paitazoglou C, wt al. Euro. Interv 2019

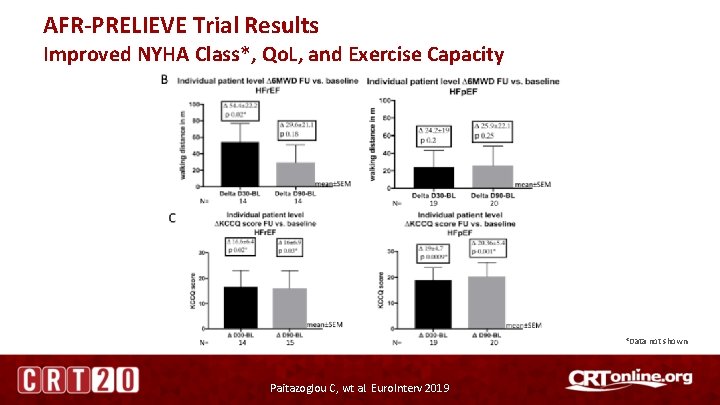
AFR-PRELIEVE Trial Results Improved NYHA Class*, Qo. L, and Exercise Capacity *Data not shown Paitazoglou C, wt al. Euro. Interv 2019

Completed Interatrial Shunt Pilot Studies REDUCE LAP HF & HF I, V-Wave Pilot Study, Occlutech PRELIEVE-HF • Striking consistency across multiple devices / trials demonstrating: • High implant success rates • Excellent procedural and device safety • Improvements in: • NYHA Class Ranking • Quality of Life Score • 6 -Minute Hall Walk Distance • Low rates of mortality and heart failure hospitalization, suggesting an impact on clinical outcomes • REDUCE LAP HF II and RELIEVE-HF pivotal trials ongoing