Atri Clip PRO 2 TM Device Product Overview






![Thank you for your attention [ 11 ] Thank you for your attention [ 11 ]](https://slidetodoc.com/presentation_image/156b5e59bae92cf8c6d211d7c720a205/image-11.jpg)

- Slides: 12

Atri. Clip PRO 2 TM Device Product Overview MKT-2387 A-G

LAA Management Guidelines Society Recommendation STS (2017) “It is REASONABLE to perform left atrial appendage excision or exclusion in conjunction with surgical ablation for AF for longitudinal thromboembolic morbidity prevention. (Class IIA, Level C limited data)” “At the time of concomitant cardiac operations in patients with AF, it is REASONABLE to surgically manage the left atrial appendage for longitudinal thromboembolic morbidity prevention. (Class IIA, Level C expert opinion)” ESC (2016) “LAA occlusion may be considered for stroke prevention in patients with AF and contra-indications for long-term anticoagulant treatment (Class IIb, Level B)”. “Surgical occlusion or exclusion of the LAA may be considered for stroke prevention in patients with AF undergoing cardiac surgery (Class IIb, Level B)”. “Surgical occlusion or exclusion of the LAA may be considered for stroke prevention in patients undergoing thoracoscopic AF surgery (Class IIb, Level B)”. UK NICE (2014) "Consider LAA occlusion if anticoagulation is contraindicated or not tolerated and discuss the benefits and risks of LAAO with patient. " EJCTS (2013) "We conclude that there has been no proven benefit of surgical LAA exclusion in terms of stroke reduction or mortality benefit… If exclusion is contemplated, devices designed for appendage exclusion should be used rather than a cut-and-sew or stapling technique. " [ 2 ] MKT-2387 A-G

Atri. Clip Pro 2 Device - Designed to Simplify the Approach to LAA Exclusion [ 3 ] MKT-2386 A-G

Atri. Clip Pro 2 Device - Designed to Simplify the Approach to LAA Exclusion • Applied epicardially not in the circulating blood during stand-alone approach • Applicable regardless of LAA morphology 1 • Addresses mechanical aspect of LAA • Over 100. 000 implants worldwide • Does not mandate any post-operative systemic anticoagulant therapy 1 Do not use on LAA <29 mm or >50 mm or <1 mm wall thickness [ 4 ] MKT-2386 A-G

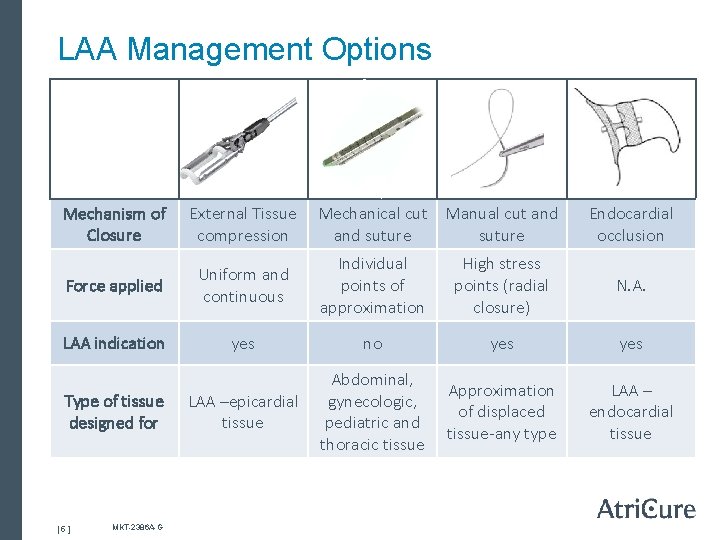
LAA Management Options Mechanism of Closure External Tissue compression Force applied Uniform and continuous Individual points of approximation High stress points (radial closure) N. A. LAA indication yes no yes LAA –epicardial tissue Abdominal, gynecologic, pediatric and thoracic tissue Approximation of displaced tissue-any type LAA – endocardial tissue Type of tissue designed for [ 5 ] MKT-2386 A-G Mechanical cut Manual cut and suture Endocardial occlusion

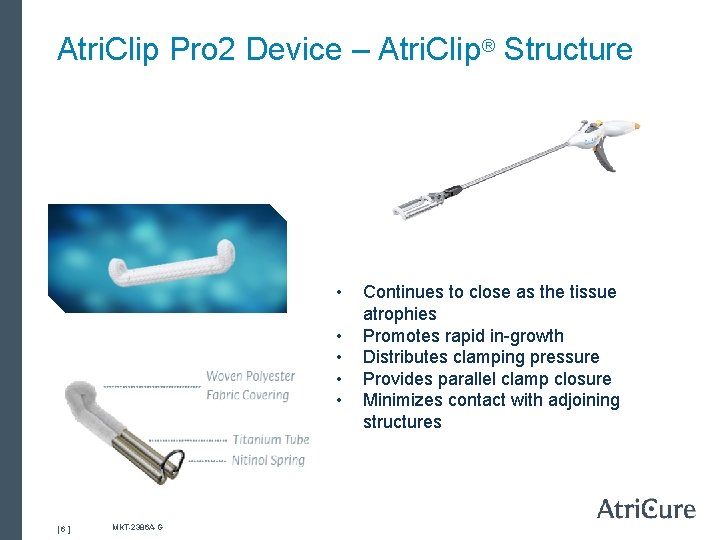
Atri. Clip Pro 2 Device – Atri. Clip® Structure • • • [ 6 ] MKT-2386 A-G Continues to close as the tissue atrophies Promotes rapid in-growth Distributes clamping pressure Provides parallel clamp closure Minimizes contact with adjoining structures

Atri. Clip Pro 2 Device – Easy of Use 12 mm Diameter End Effector • Allows for enhanced visualization in the chest cavity and/or transverse sinus • Fits 12 mm trocar • Can be used in totally thoracoscopic procedures [ 7 ] MKT-2386 A-G

Atri. Clip Pro 2 Device – Easy of Use Active Articulation Levers • Remotely/actively control the end effector for +/- 30 degree pitch and yaw [ 8 ] MKT-2386 A-G

Atri. Clip Pro 2 Device – Performance LAA Mechanical Isolation In preclinical and clinical studies, the Atri. Clip® device securely sealed the LAA orifice resulting in a smooth endothelial tissue surface within 90 days Ailawadi G, Gerdisch MW, Harvey RL, et al. Exclusion of the left atrial appendage with a novel device: early results of a multicenter trial. J Thorac Cardiovasc Surg 2011; 142: 1002– 9 [ 9 ] MKT-2386 A-G • • • Prospective, Multicenter, Non-randomized (N= 70 patients) Open chest during concomitant cardiac surgery 70/70 clips successfully implanted 67/70 complete exclusion confirmed by intra-op TEE (>95%) 60/61 exclusion confirmed by 3 month by CT scan (> 98%) • • No device or clip procedure related adverse events reported No evidence of clip migration on any CT evaluation

Atri. Clip Pro 2 Device – Performance Safety The Atri. Clip device provides safe and atraumatic exclusion of the LAA during open and endoscopic cardiac surgery Emmert et al. Safe, Effective and Durable Epicardial Left Atrial Appendage Clip Occlusion in Patients with Atrial Fibrillation Undergoing Cardiac Surgery: First Long-Term Results from a Prospective Device Trial, European Journal of Cardio-Thoracic Surgery 2013 “Herein we report the first long-term outcome data on epicardial LAA clip application during open heart surgery. In addition to being 100% effective and safe in the short term , the results of this prospective device trial demonstrate the durable occlusion of the LAA for over 3 years with excellent clinical outcomes. Importantly, during follow-up, no strokes occurred anticoagulation could be often discontinued, most often decided by general practitioners and referring cardiologists. “ [ 10 ] MKT-2386 A-G
![Thank you for your attention 11 Thank you for your attention [ 11 ]](https://slidetodoc.com/presentation_image/156b5e59bae92cf8c6d211d7c720a205/image-11.jpg)
Thank you for your attention [ 11 ]

Atri. Clip PRO 2 Device - Indication for Use Indication for use • The Atri. Clip LAA Exclusion System is indicated for the occlusion of the heart’s left atrial appendage Contraindications • Tubal occlusion • Known allergy to Nickel (Nitinol) Warnings • Do not attempt to reposition or remove the device after deployment • Do not use this device if the patient has sensitivity to nickel Precautions • Pre-op or intra-op confirmation that no thrombus is present in appendage prior to clip placement is required. As this may not be routinely performed in all procedures, remind surgeon of this procedural step in advance • Do not use on LAA <29 mm or >50 mm or <1 mm wall thickness MRI • MRI Conditional due to artifacts • Less than 3 Tesla [ 12 ] MKT-2386 A-G