Atoms to Minerals Section 3 1 Atomic Structure


























- Slides: 66

Atoms to Minerals

Section 3. 1: Atomic Structure of Matter What is matter?

Section 3. 1: Atomic Structure of Matter is anything that has mass and volume

Section 3. 1: Atomic Structure of Matter • We can classify matter by using the Periodic Table • 3 Large Groups of elements on the periodic table

Section 3. 1: Atomic Structure of Matter • 1. Metal: shiny, ductile, conducts electricity • 2. Non-metal: dull, does not conduct electricity • 3. Metalloid: Shares properties of both metal and non-metal • Elements are classified according to their place on the Periodic Table.

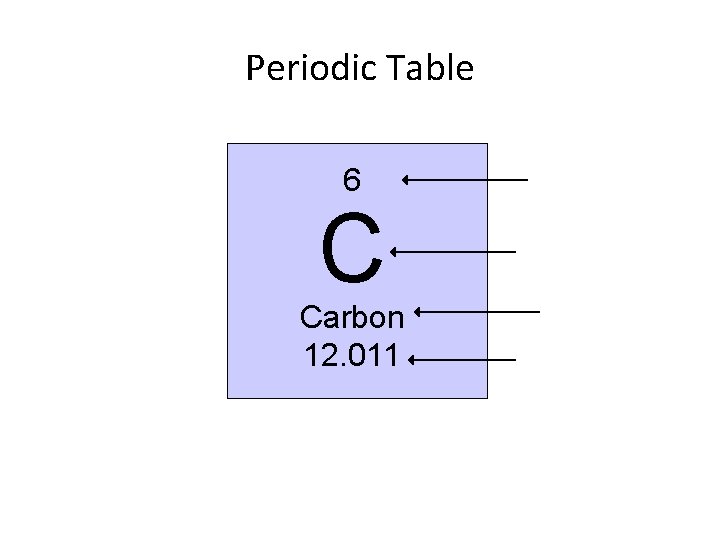
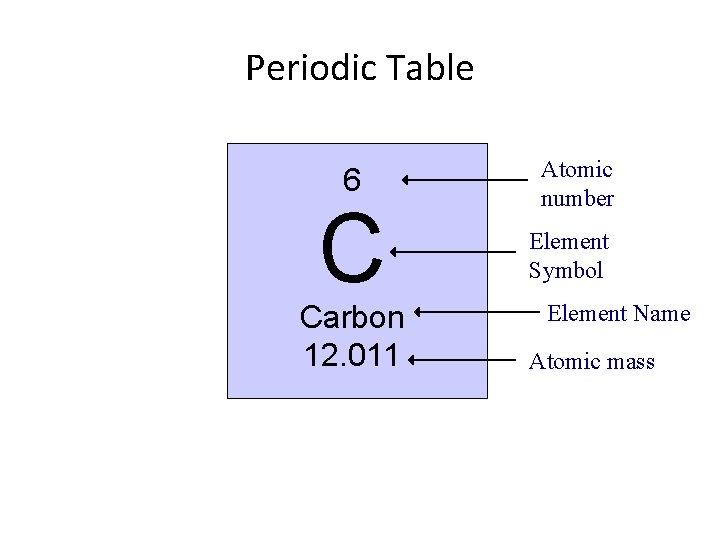
Periodic Table 6 C Carbon 12. 011

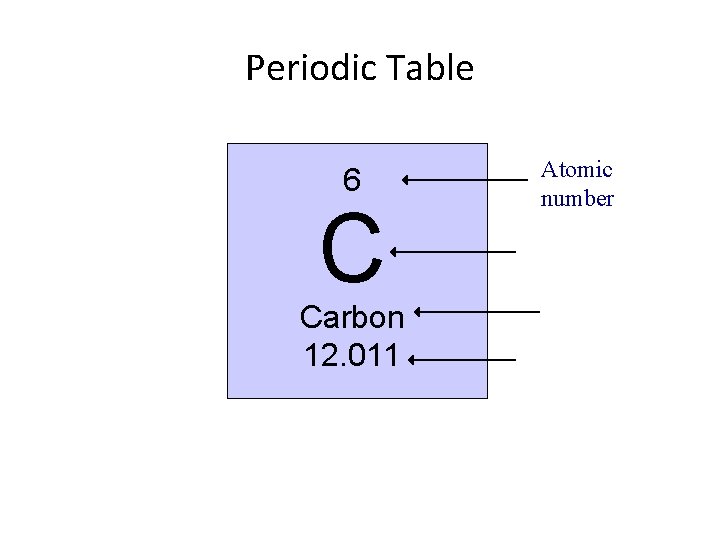
Periodic Table 6 C Carbon 12. 011 Atomic number

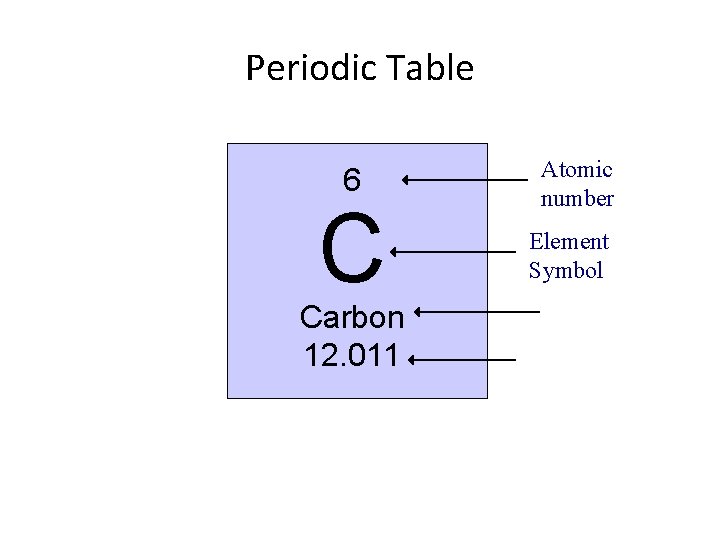
Periodic Table 6 C Carbon 12. 011 Atomic number Element Symbol

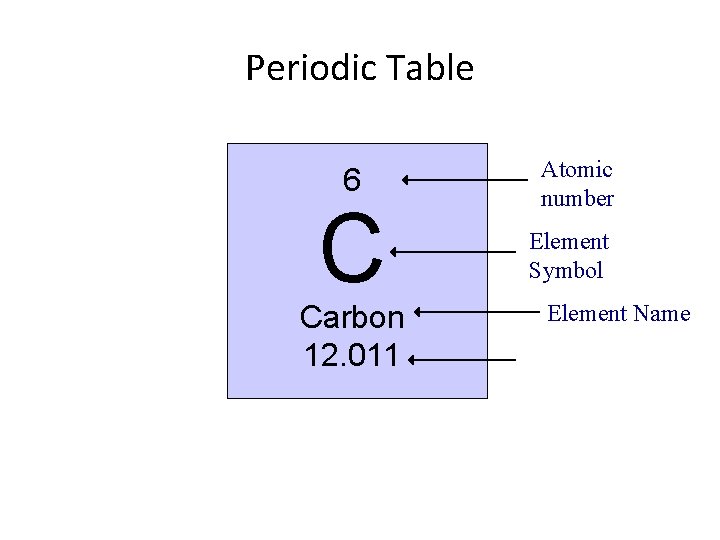
Periodic Table 6 C Carbon 12. 011 Atomic number Element Symbol Element Name

Periodic Table 6 C Carbon 12. 011 Atomic number Element Symbol Element Name Atomic mass

Section 3. 1: Atomic Structure of Matter Structure of the Atom • Nucleus: This contains positively charged protons and neutrally charged neutrons. • Electrons: These surround the nucleus in an electron cloud and are negatively charged.

Section 3. 1: Atomic Structure of Matter Structure of the Atom • The atomic number, or identity of the atom, is determined by the number of PROTONS!

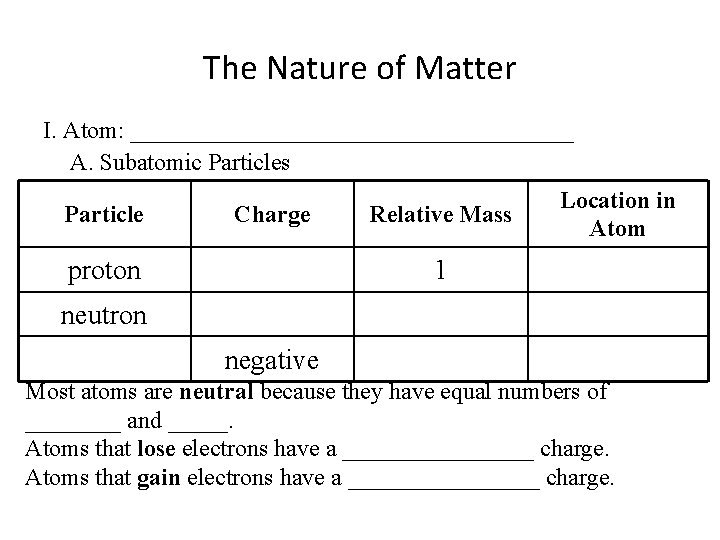
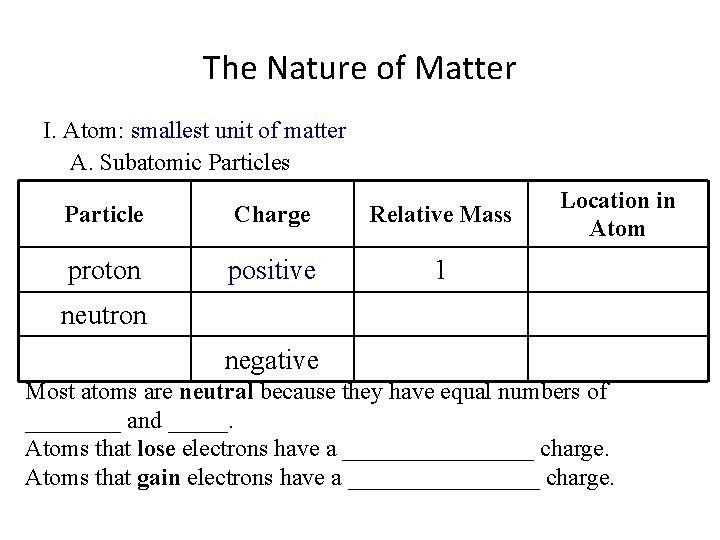
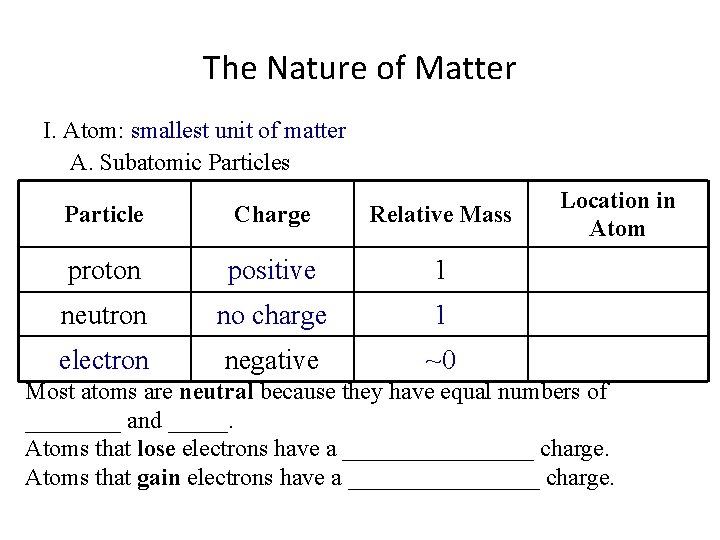
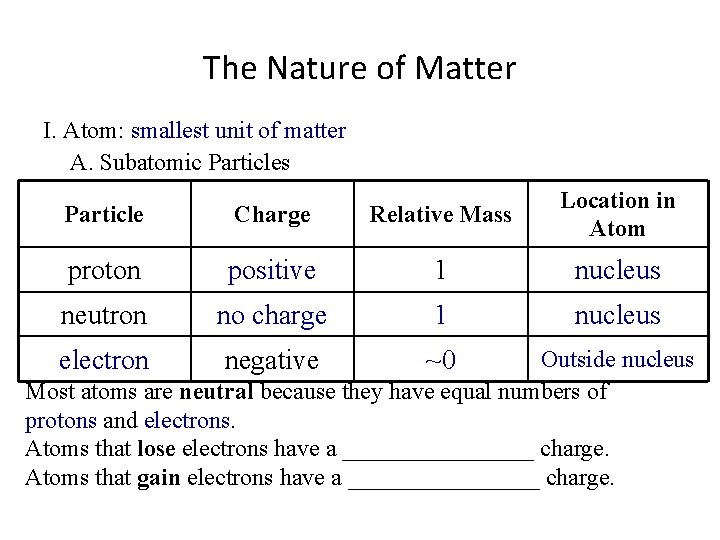
The Nature of Matter I. Atom: ___________________ A. Subatomic Particles Particle Charge proton Relative Mass Location in Atom 1 neutron negative Most atoms are neutral because they have equal numbers of ____ and _____. Atoms that lose electrons have a ________ charge. Atoms that gain electrons have a ________ charge.

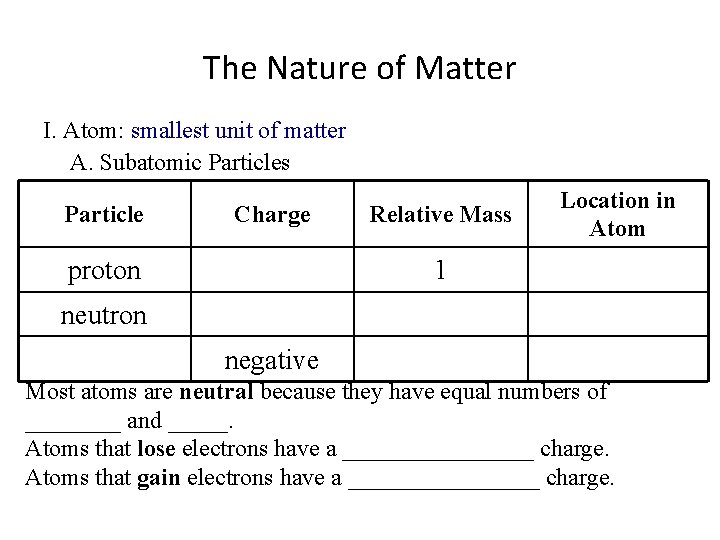
The Nature of Matter I. Atom: smallest unit of matter A. Subatomic Particles Particle Charge proton Relative Mass Location in Atom 1 neutron negative Most atoms are neutral because they have equal numbers of ____ and _____. Atoms that lose electrons have a ________ charge. Atoms that gain electrons have a ________ charge.

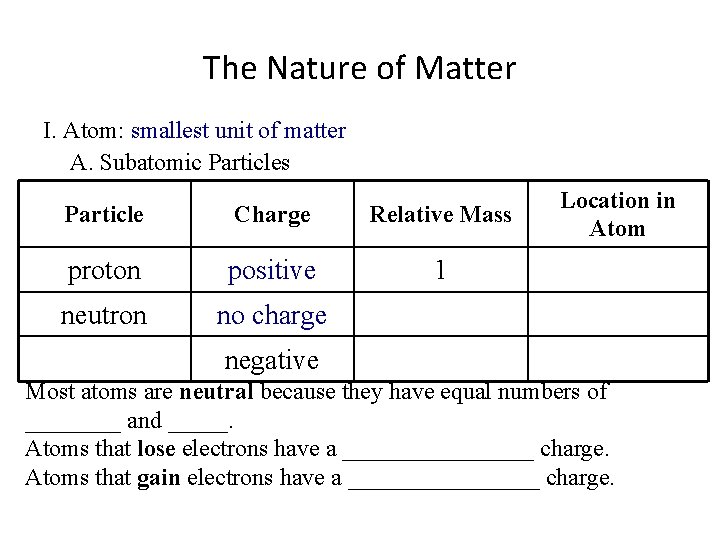
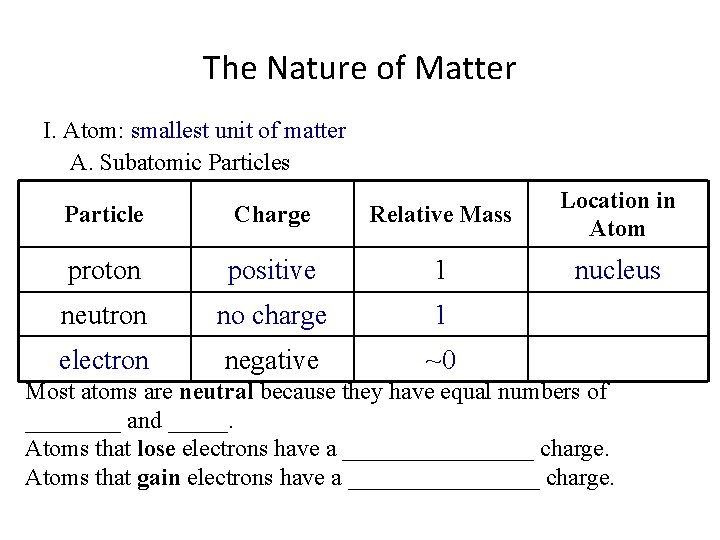
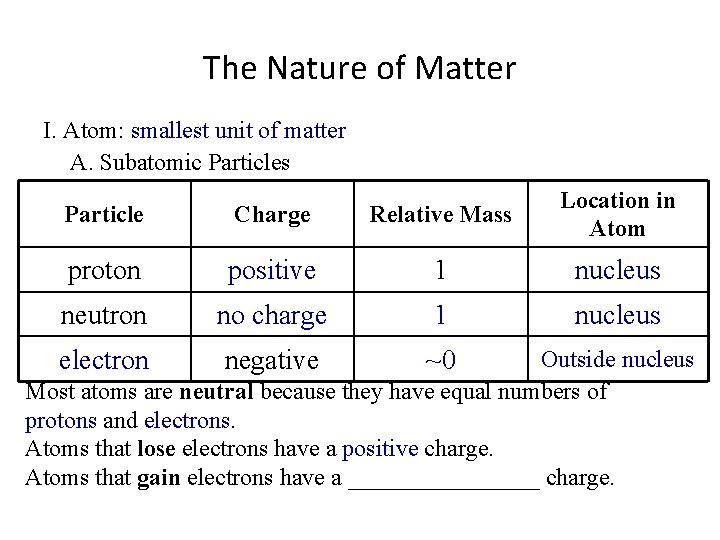
The Nature of Matter I. Atom: smallest unit of matter A. Subatomic Particles Particle Charge Relative Mass proton positive 1 Location in Atom neutron negative Most atoms are neutral because they have equal numbers of ____ and _____. Atoms that lose electrons have a ________ charge. Atoms that gain electrons have a ________ charge.

The Nature of Matter I. Atom: smallest unit of matter A. Subatomic Particles Particle Charge Relative Mass proton positive 1 neutron no charge Location in Atom negative Most atoms are neutral because they have equal numbers of ____ and _____. Atoms that lose electrons have a ________ charge. Atoms that gain electrons have a ________ charge.

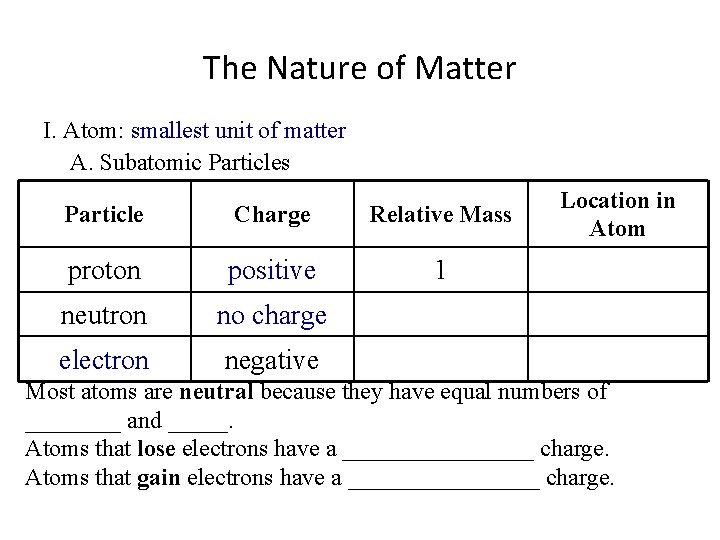
The Nature of Matter I. Atom: smallest unit of matter A. Subatomic Particles Particle Charge Relative Mass proton positive 1 neutron no charge electron negative Location in Atom Most atoms are neutral because they have equal numbers of ____ and _____. Atoms that lose electrons have a ________ charge. Atoms that gain electrons have a ________ charge.

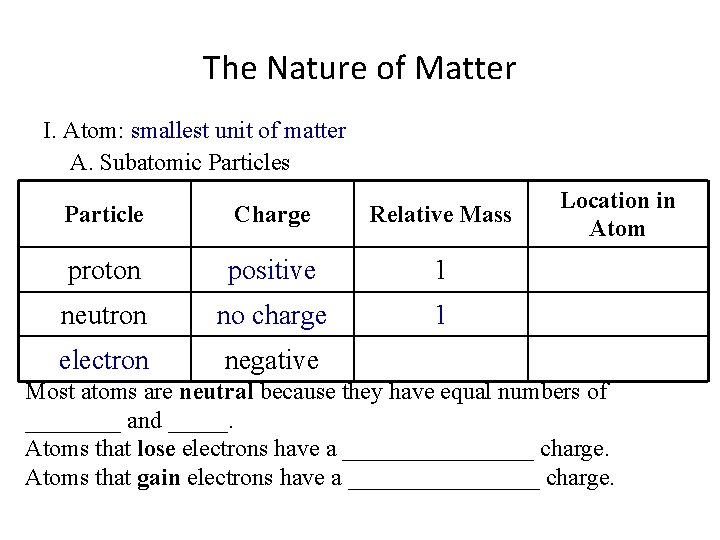
The Nature of Matter I. Atom: smallest unit of matter A. Subatomic Particles Particle Charge Relative Mass proton positive 1 neutron no charge 1 electron negative Location in Atom Most atoms are neutral because they have equal numbers of ____ and _____. Atoms that lose electrons have a ________ charge. Atoms that gain electrons have a ________ charge.

The Nature of Matter I. Atom: smallest unit of matter A. Subatomic Particles Particle Charge Relative Mass proton positive 1 neutron no charge 1 electron negative ~0 Location in Atom Most atoms are neutral because they have equal numbers of ____ and _____. Atoms that lose electrons have a ________ charge. Atoms that gain electrons have a ________ charge.

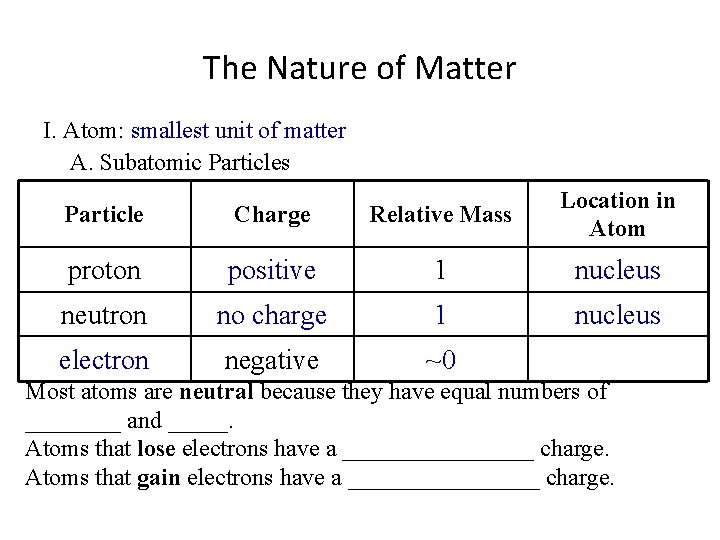
The Nature of Matter I. Atom: smallest unit of matter A. Subatomic Particles Particle Charge Relative Mass Location in Atom proton positive 1 nucleus neutron no charge 1 electron negative ~0 Most atoms are neutral because they have equal numbers of ____ and _____. Atoms that lose electrons have a ________ charge. Atoms that gain electrons have a ________ charge.

The Nature of Matter I. Atom: smallest unit of matter A. Subatomic Particles Particle Charge Relative Mass Location in Atom proton positive 1 nucleus neutron no charge 1 nucleus electron negative ~0 Most atoms are neutral because they have equal numbers of ____ and _____. Atoms that lose electrons have a ________ charge. Atoms that gain electrons have a ________ charge.

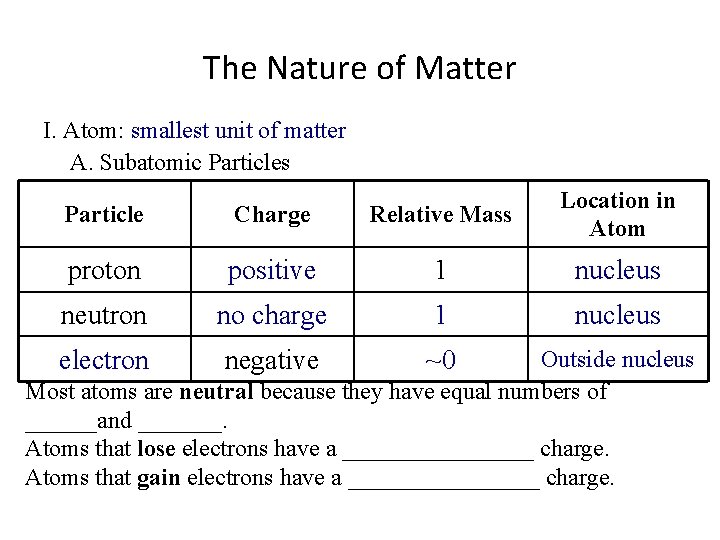
The Nature of Matter I. Atom: smallest unit of matter A. Subatomic Particles Particle Charge Relative Mass Location in Atom proton positive 1 nucleus neutron no charge 1 nucleus electron negative ~0 Outside nucleus Most atoms are neutral because they have equal numbers of ______and _______. Atoms that lose electrons have a ________ charge. Atoms that gain electrons have a ________ charge.

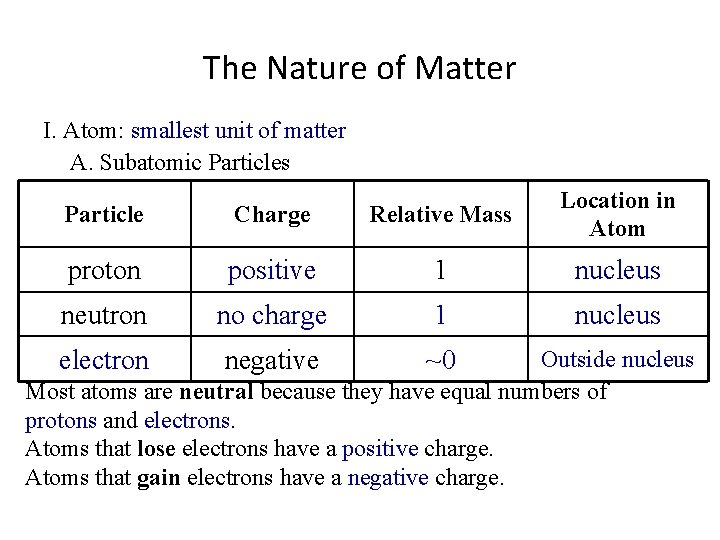
The Nature of Matter I. Atom: smallest unit of matter A. Subatomic Particles Particle Charge Relative Mass Location in Atom proton positive 1 nucleus neutron no charge 1 nucleus electron negative ~0 Outside nucleus Most atoms are neutral because they have equal numbers of protons and electrons. Atoms that lose electrons have a ________ charge. Atoms that gain electrons have a ________ charge.

The Nature of Matter I. Atom: smallest unit of matter A. Subatomic Particles Particle Charge Relative Mass Location in Atom proton positive 1 nucleus neutron no charge 1 nucleus electron negative ~0 Outside nucleus Most atoms are neutral because they have equal numbers of protons and electrons. Atoms that lose electrons have a positive charge. Atoms that gain electrons have a ________ charge.

The Nature of Matter I. Atom: smallest unit of matter A. Subatomic Particles Particle Charge Relative Mass Location in Atom proton positive 1 nucleus neutron no charge 1 nucleus electron negative ~0 Outside nucleus Most atoms are neutral because they have equal numbers of protons and electrons. Atoms that lose electrons have a positive charge. Atoms that gain electrons have a negative charge.


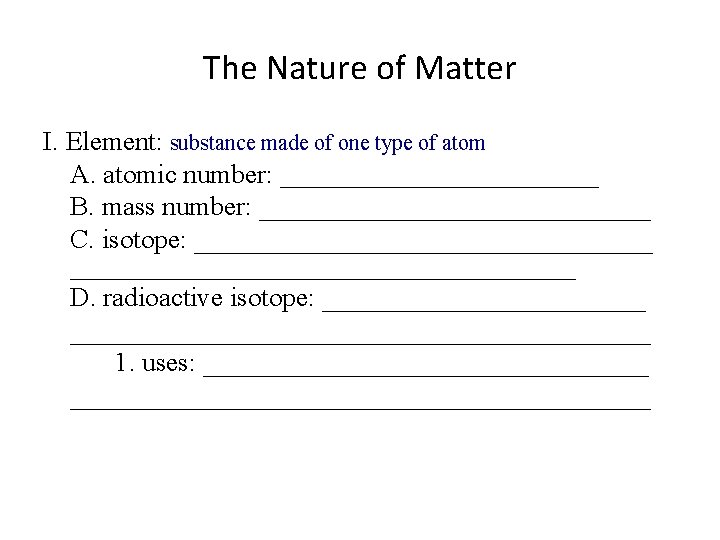
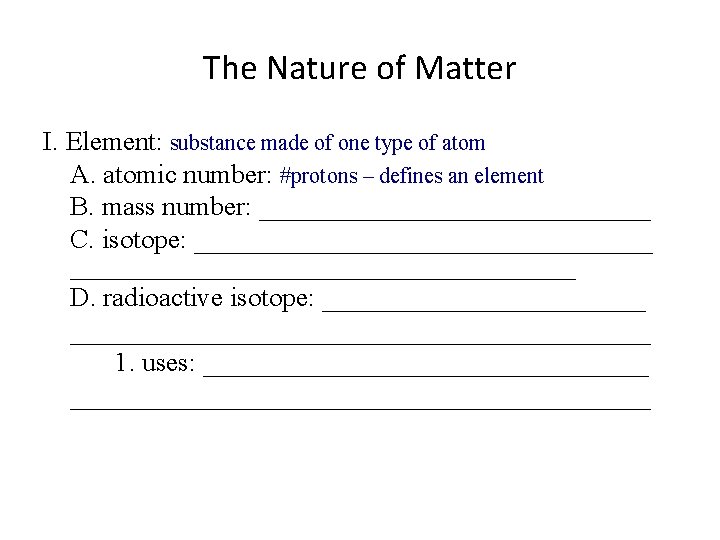
The Nature of Matter I. Element: substance made of one type of atom A. atomic number: _______________ B. mass number: _______________ C. isotope: ________________________________________ D. radioactive isotope: __________________________________ 1. uses: ______________________________________

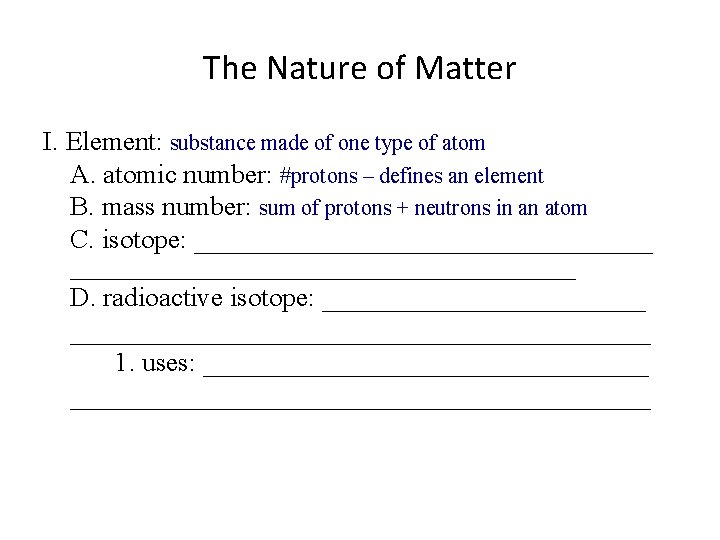
The Nature of Matter I. Element: substance made of one type of atom A. atomic number: #protons – defines an element B. mass number: _______________ C. isotope: ________________________________________ D. radioactive isotope: __________________________________ 1. uses: ______________________________________

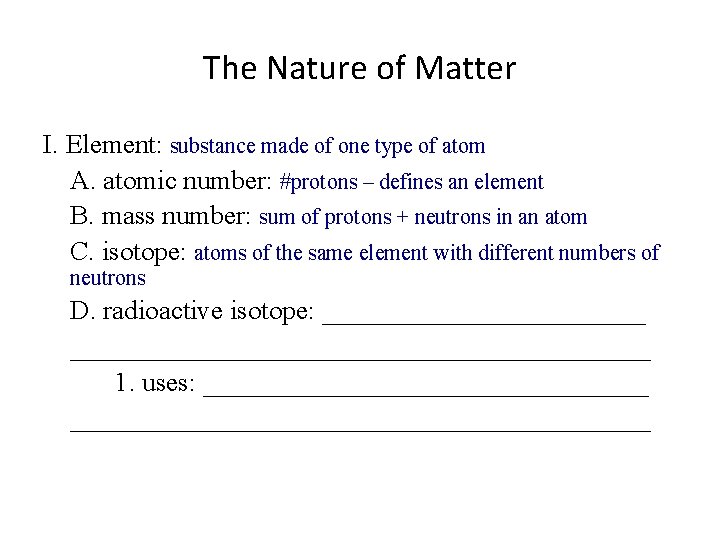
The Nature of Matter I. Element: substance made of one type of atom A. atomic number: #protons – defines an element B. mass number: sum of protons + neutrons in an atom C. isotope: ________________________________________ D. radioactive isotope: __________________________________ 1. uses: ______________________________________

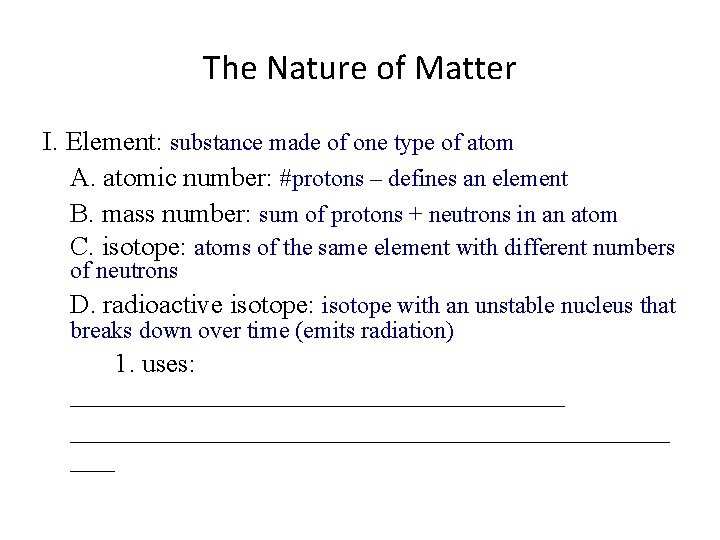
The Nature of Matter I. Element: substance made of one type of atom A. atomic number: #protons – defines an element B. mass number: sum of protons + neutrons in an atom C. isotope: atoms of the same element with different numbers of neutrons D. radioactive isotope: __________________________________ 1. uses: ______________________________________

The Nature of Matter I. Element: substance made of one type of atom A. atomic number: #protons – defines an element B. mass number: sum of protons + neutrons in an atom C. isotope: atoms of the same element with different numbers of neutrons D. radioactive isotope: isotope with an unstable nucleus that breaks down over time (emits radiation) 1. uses: ________________________________________

The Nature of Matter I. Element: substance made of one type of atom A. atomic number: #protons – defines an element B. mass number: sum of protons + neutrons in an atom C. isotope: atoms of the same element with different numbers of neutrons D. radioactive isotope: isotope with an unstable nucleus that breaks down over time (emits radiation) 1. uses: dating fossils, diagnosing and treating diseases, killing bacteria in food/ on surgical equipment

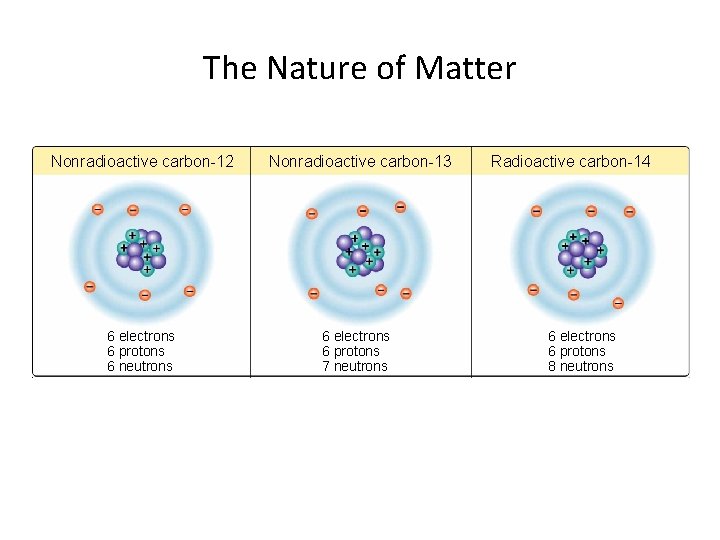
The Nature of Matter Nonradioactive carbon-12 6 electrons 6 protons 6 neutrons Nonradioactive carbon-13 6 electrons 6 protons 7 neutrons Radioactive carbon-14 6 electrons 6 protons 8 neutrons

Section 3. 1: Atomic Structure of Matter • • Structure of the Atom Isotopes are atoms with usually more neutrons than protons. The mass number equals the sum of protons plus neutrons.

Section 3. 2: Composition of Minerals • • Structure of the Atom A compound is a substance with two or more elements. Example is table salt or Sodium Chloride (Na. Cl)

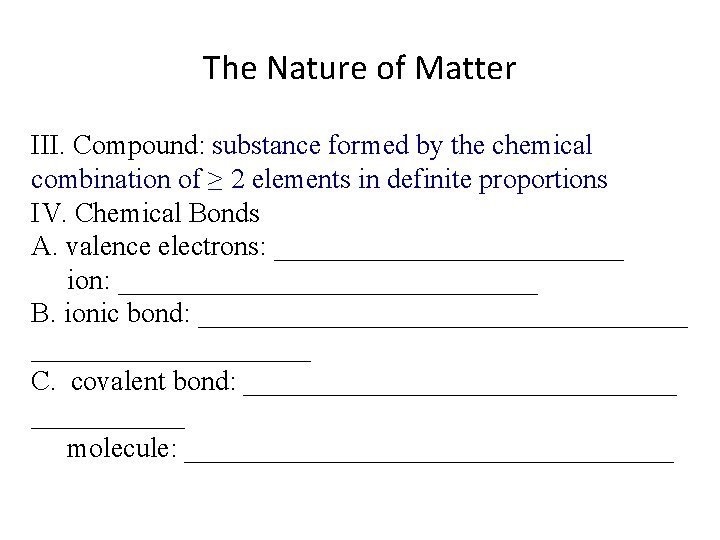
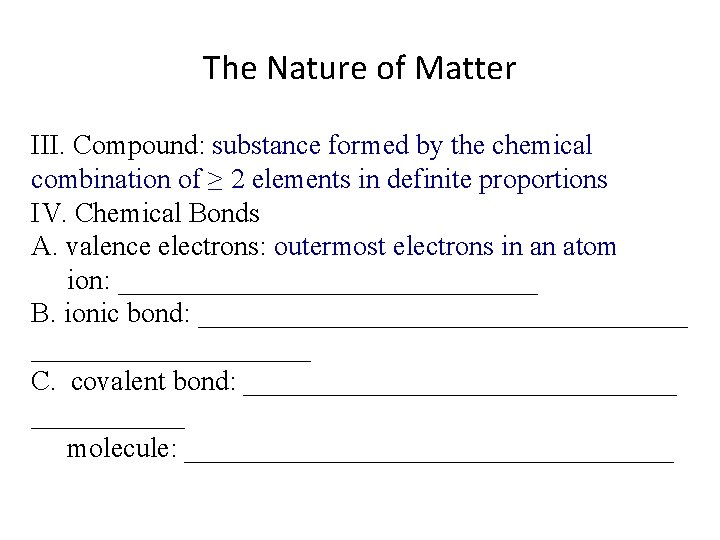
The Nature of Matter III. Compound: ________________________________________ IV. Chemical Bonds A. valence electrons: _____________ ion: _______________ B. ionic bond: __________________ C. covalent bond: ________________ molecule: __________________

The Nature of Matter III. Compound: substance formed by the chemical combination of ≥ 2 elements in definite proportions IV. Chemical Bonds A. valence electrons: _____________ ion: _______________ B. ionic bond: __________________ C. covalent bond: ________________ molecule: __________________

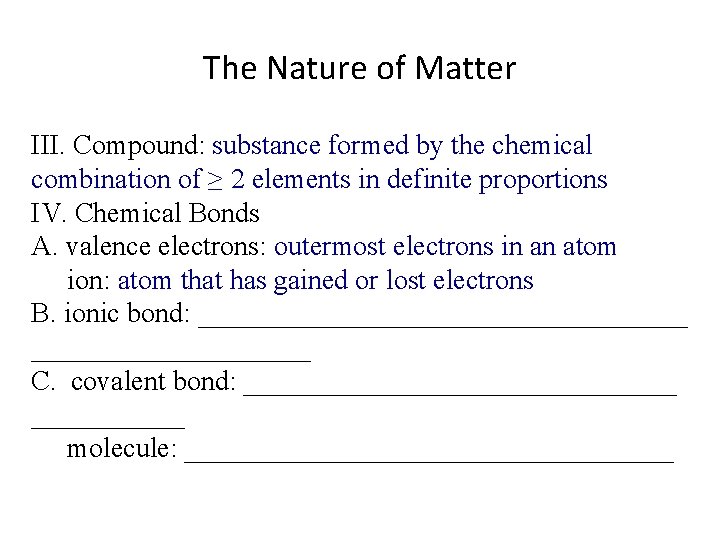
The Nature of Matter III. Compound: substance formed by the chemical combination of ≥ 2 elements in definite proportions IV. Chemical Bonds A. valence electrons: outermost electrons in an atom ion: _______________ B. ionic bond: __________________ C. covalent bond: ________________ molecule: __________________

The Nature of Matter III. Compound: substance formed by the chemical combination of ≥ 2 elements in definite proportions IV. Chemical Bonds A. valence electrons: outermost electrons in an atom ion: atom that has gained or lost electrons B. ionic bond: __________________ C. covalent bond: ________________ molecule: __________________

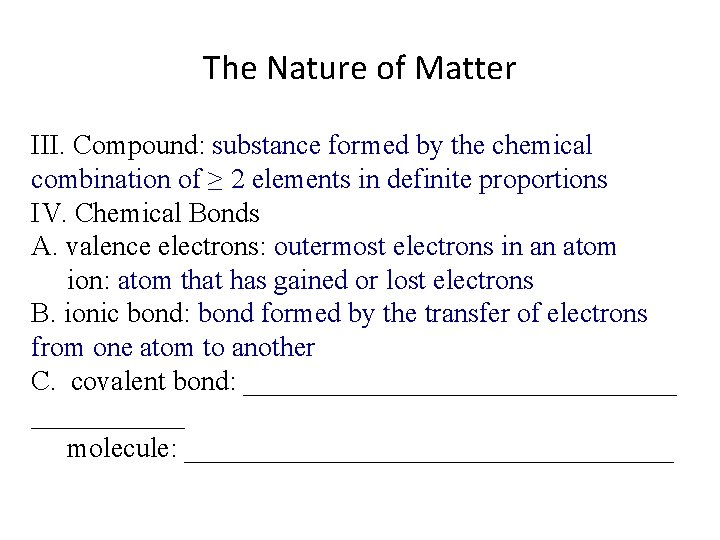
The Nature of Matter III. Compound: substance formed by the chemical combination of ≥ 2 elements in definite proportions IV. Chemical Bonds A. valence electrons: outermost electrons in an atom ion: atom that has gained or lost electrons B. ionic bond: bond formed by the transfer of electrons from one atom to another C. covalent bond: ________________ molecule: __________________

The Nature of Matter III. Compound: substance formed by the chemical combination of ≥ 2 elements in definite proportions IV. Chemical Bonds A. valence electrons: outermost electrons in an atom ion: atom that has gained or lost electrons B. ionic bond: bond formed by the transfer of electrons from one atom to another C. covalent bond: bond formed when atoms share electrons molecule: __________________

The Nature of Matter III. Compound: substance formed by the chemical combination of ≥ 2 elements in definite proportions IV. Chemical Bonds A. valence electrons: outermost electrons in an atom ion: atom that has gained or lost electrons B. ionic bond: bond formed by the transfer of electrons from one atom to another C. covalent bond: bond formed when atoms share electrons molecule: group of atoms covalently bonded together

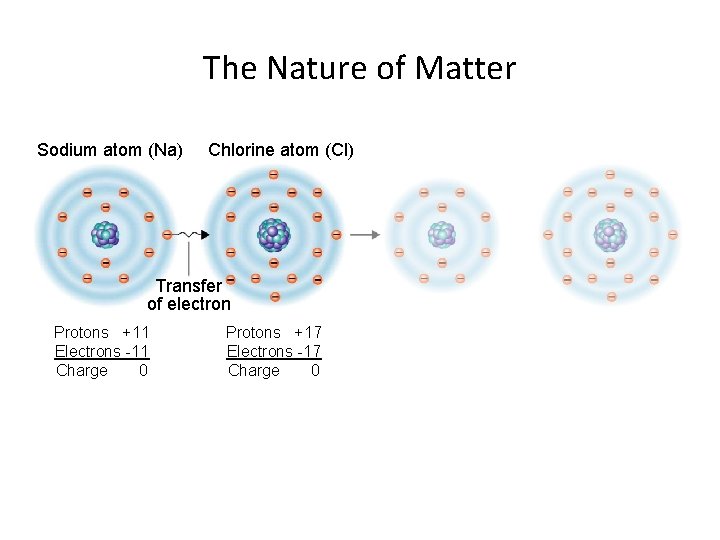
The Nature of Matter Sodium atom (Na) Chlorine atom (Cl) Transfer of electron Protons +11 Electrons -11 Charge 0 Protons +17 Electrons -17 Charge 0

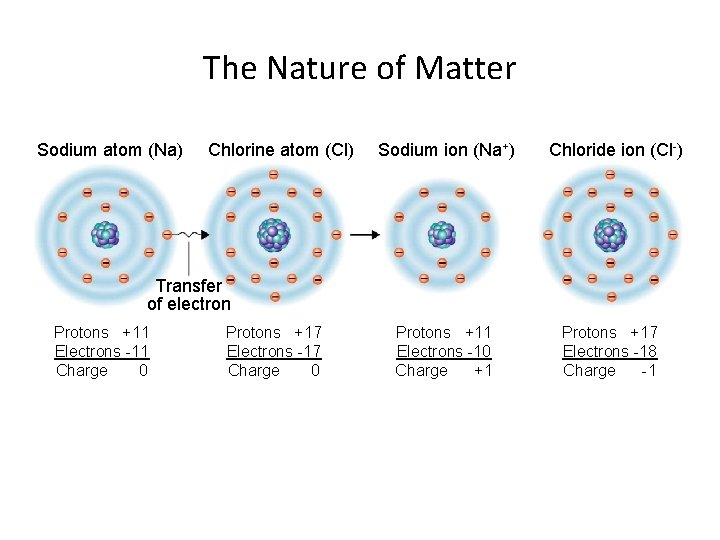
The Nature of Matter Sodium atom (Na) Chlorine atom (Cl) Sodium ion (Na+) Chloride ion (Cl-) Transfer of electron Protons +11 Electrons -11 Charge 0 Protons +17 Electrons -17 Charge 0 Protons +11 Electrons -10 Charge +1 Protons +17 Electrons -18 Charge -1

Section 3. 2: Composition of Minerals Bonding of Atoms • Ions are formed when the number of electrons is either more or less than the number of protons.

Section 3. 2: Composition of Minerals • • Bonding of Atoms A covalent bond is when two atoms share electrons. A molecule is formed by covalent bonding of two or more atoms.

Section 3. 2: Composition of Minerals Bonding of Atoms • An ionic bond is when atoms are held together by electrical attraction.

Section 3. 2: Composition of Minerals What is a mineral?

Section 3. 2: Composition of Minerals 1. It occurs naturally. 2. It is solid. 3. It has a chemical composition. 4. It’s atoms are arranged in an orderly pattern (crystal structure). 5. It is inorganic (not alive).

Section 3. 2: Composition of Minerals How do minerals form?

Section 3. 2: Composition of Minerals How do minerals form? 1. By the Crystallization of Magma as it cools. Crystals form over long periods of slow cooling of magma. 2. By the pressure process as minerals are converted to other minerals by great amounts of heat and pressure. 3. By the evaporation of mineral-rich water. This is how geodes and the Cavern of Crystal Giants formed.

Section 3. 3: Structure of Minerals Why should we care about minerals? What do we use them for and what is our impact on the planet by using them?

Section 3. 3: Structure of Minerals What is the basic chemistry of the Earth’s Crust?

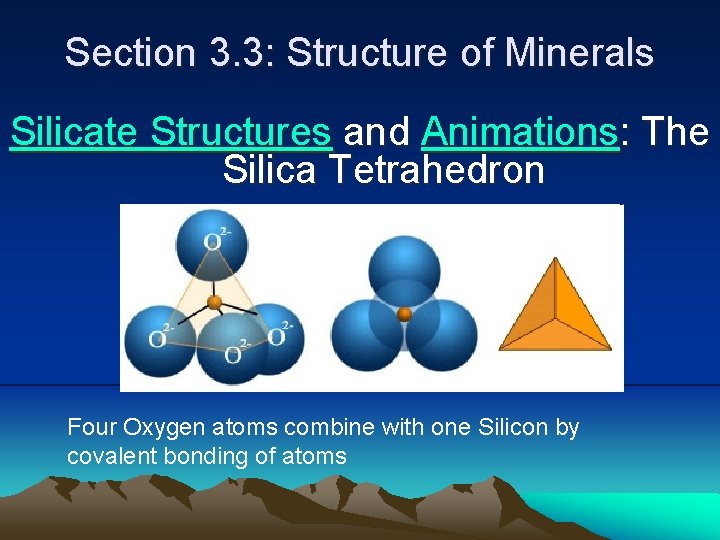
Section 3. 3: Structure of Minerals Silicate Structures and Animations: The Silica Tetrahedron Four Oxygen atoms combine with one Silicon by covalent bonding of atoms

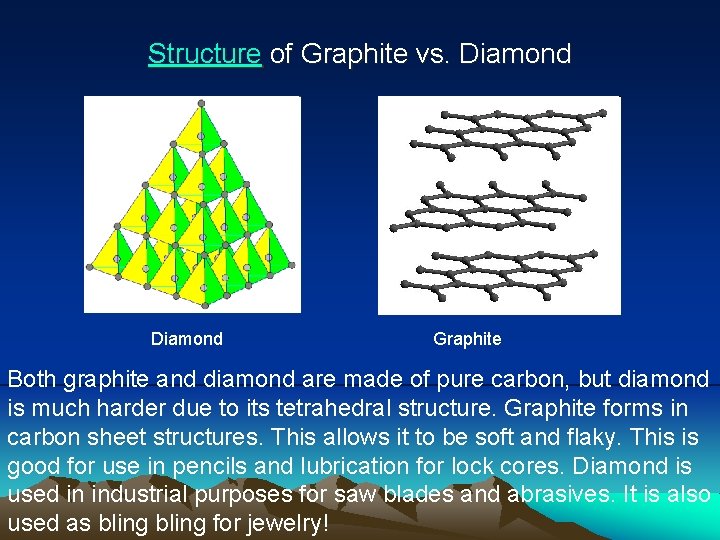
Structure of Graphite vs. Diamond Graphite Both graphite and diamond are made of pure carbon, but diamond is much harder due to its tetrahedral structure. Graphite forms in carbon sheet structures. This allows it to be soft and flaky. This is good for use in pencils and lubrication for lock cores. Diamond is used in industrial purposes for saw blades and abrasives. It is also used as bling for jewelry!

Structure of Graphite vs. Diamond Graphite Both graphite and diamond are made of pure carbon, but diamond is much harder due to its tetrahedral structure. Graphite forms in carbon sheet structures. This allows it to be soft and flaky. This is good for use in pencils and lubrication for lock cores. Diamond is used in industrial purposes for saw blades and abrasives. It is also used as bling for jewelry!

Identifying Minerals On to the Mineral Identification Lab!

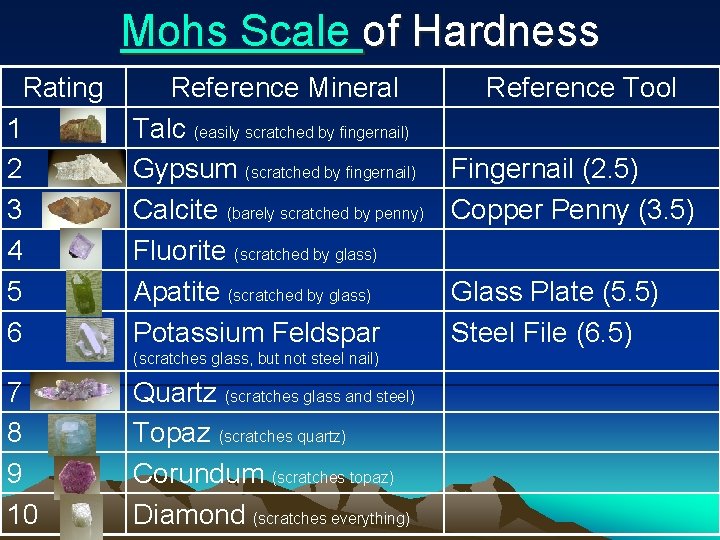
Mohs Scale of Hardness Rating 1 2 3 4 5 6 Reference Mineral Talc (easily scratched by fingernail) Gypsum (scratched by fingernail) Calcite (barely scratched by penny) Fluorite (scratched by glass) Apatite (scratched by glass) Potassium Feldspar (scratches glass, but not steel nail) 7 8 9 10 Quartz (scratches glass and steel) Topaz (scratches quartz) Corundum (scratches topaz) Diamond (scratches everything) Reference Tool Fingernail (2. 5) Copper Penny (3. 5) Glass Plate (5. 5) Steel File (6. 5)

Mineral Groups Silicates • 90% of the Minerals in Earth’s crust are silicates. • A silicate is a compound of Silicon, Oxygen, and one or more metallic elements. Quartz (Si. O 2) Orthoclase Feldspar (KAl. Si 3 O 8)

Mineral Groups Carbonates • Positive metal ion combined with a negative Carbonate (CO 3 2 -) ion. • Fizzes with Hydrochloric Acid Calcite (Ca. CO 3) Malachite (Ca. CO 3) Dolomite Ca. Mg(CO 3)2

Mineral Groups Halides • Group of minerals that contains one of the halogen elements (F, Cl, Br, I) as a building block Fluorite (Ca. F 2) Halite (Na. Cl)

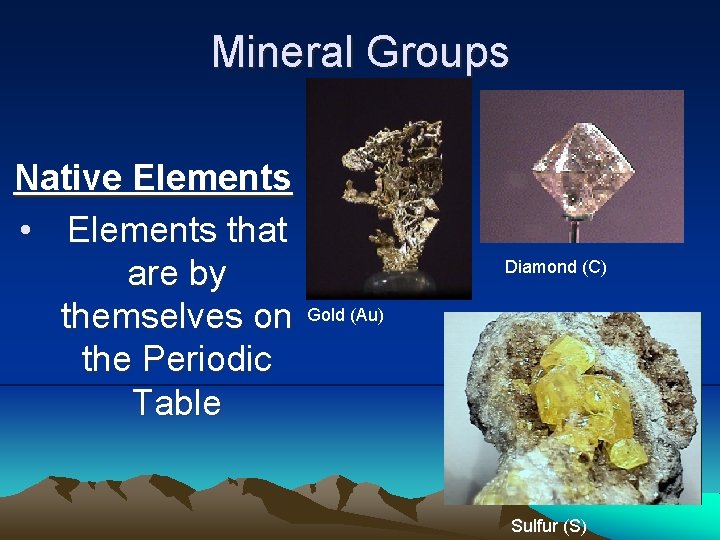
Mineral Groups Native Elements • Elements that are by themselves on the Periodic Table Diamond (C) Gold (Au) Sulfur (S)

Mineral Groups Oxides • Metal element combined with oxygen • Valuable for their economic and industrial importance Hematite (Fe 2 O 3) Magnetite (Fe 3 O 4)

Mineral Groups Sulfates • Compounds containing the sulfate group (SO 42 -) • Gypsum valuable for its industrial importance in construction (wallboard) • Barite useful as main ore of Barium (used for barium contrast for Xray machines) Gypsum (Ca. SO 4*2 H 2 O) Barite (Ba. SO 4)

Mineral Groups Sulfides • Metal element combined with sulfur. • When Hydrochloric Acid is poured on Galena, hydrogen sulfide gas (used to make stink bombs) is produced! Yuck! Pyrite (Fe. S 2) Galena (Pb. S)

Mineral Groups • Phosphates • Minerals that have phosphate (PO 4)3 - in their chemical formula • Turquoise used for centuries by Egyptians and Native Americans for jewelry Apatite is the main source of the phosphorous nutrient for plants. Your bones and teeth are made of the same substance as apatite! Turquoise (Cu. Al 6(PO 4)4(OH)8*5(H 2 O)) Apatite (Ca 5(PO 4)3(F, Cl, OH) )