Atoms The Building Blocks of Matter Chem Catalyst
Atoms: The Building Blocks of Matter

Chem Catalyst in folder 1. Which Philosopher was responsible for the term “atomos”? 2. Which “scientist” revived Democritus's ideas regarding matter? 3. Which scientist used the cathode ray tube to isolate the electron in 1897? 4. Which Philosopher replaced the correct theory of atoms and made his own using the earthly elements? 5. Give an example from the atomic history that shows how scientists build on each other’s knowledge. 6. How have scientists used indirect observation to learn about atoms? 7. How have scientists taken advantage of accidental or unexpected experimental results to create new knowledge? 8. How did the “Gold Foil’ Experiment lead to the discovery of a Nucleus at the center of an atom? 9. Why did it take so long for the neutron to be discovered? 10. What made Chadwick pursue research of the neutron?
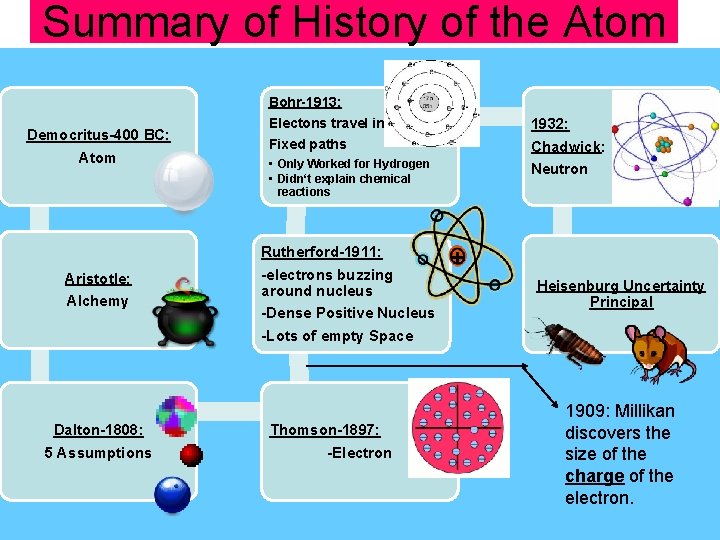
Summary of History of the Atom Bohr-1913: Democritus-400 BC: Atom Aristotle: Alchemy Dalton-1808: 5 Assumptions Electons travel in 1932: Chadwick: Neutron Fixed paths • Only Worked for Hydrogen • Didn‘t explain chemical reactions Rutherford-1911: -electrons buzzing around nucleus -Dense Positive Nucleus -Lots of empty Space Thomson-1897: -Electron + Heisenburg Uncertainty Principal 1909: Millikan discovers the size of the charge of the electron.
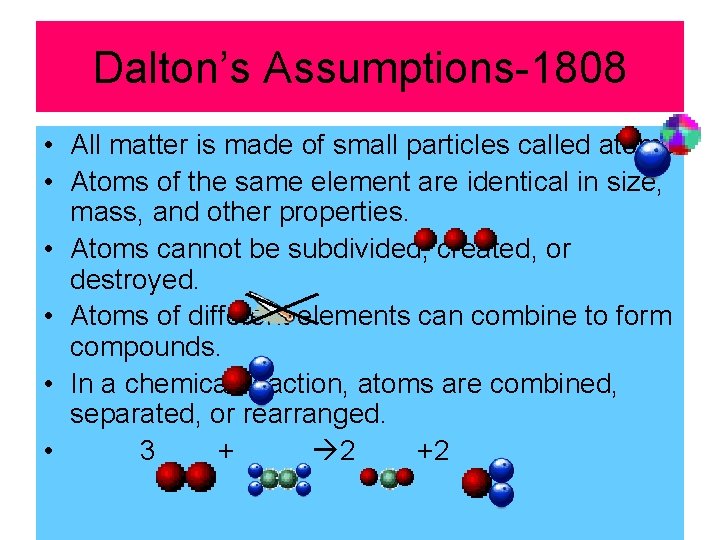
Dalton’s Assumptions-1808 • All matter is made of small particles called atoms. • Atoms of the same element are identical in size, mass, and other properties. • Atoms cannot be subdivided, created, or destroyed. • Atoms of different elements can combine to form compounds. • In a chemical reaction, atoms are combined, separated, or rearranged. • 3 + 2 +2

How to get started today: • Get a card from the table and pick an element from the periodic table (element 3 -14). Fill out your card with that elements info. • Grab a sheet from the table and cut off the edges so that it will fit in you comp book. Cut in between the words on the right to make flaps. • Hold on to these two things until instructed otherwise • Get out paper for quiz.
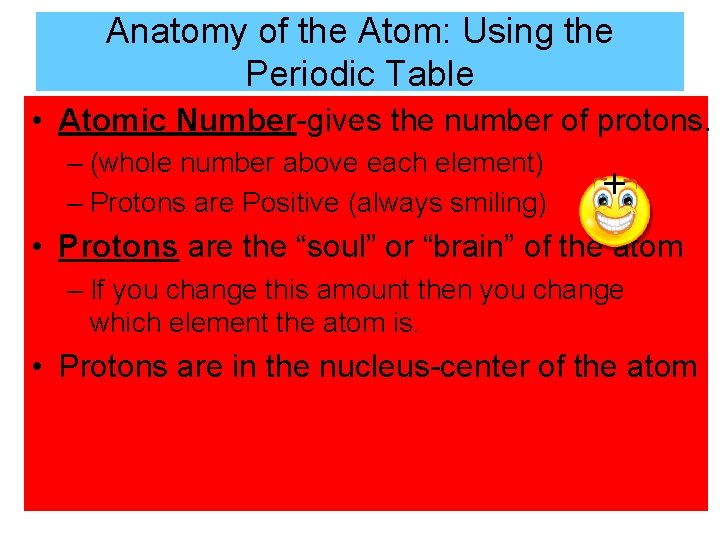
Anatomy of the Atom: Using the Periodic Table • Atomic Number-gives the number of protons. – (whole number above each element) – Protons are Positive (always smiling) + • Protons are the “soul” or “brain” of the atom – If you change this amount then you change which element the atom is. • Protons are in the nucleus-center of the atom
Anatomy of the Atom/Periodic Table • Neutrons: Particles that are in the nucleus with the protons. • Nucleus: Center of the atom. Contains Protons and Neutrons.
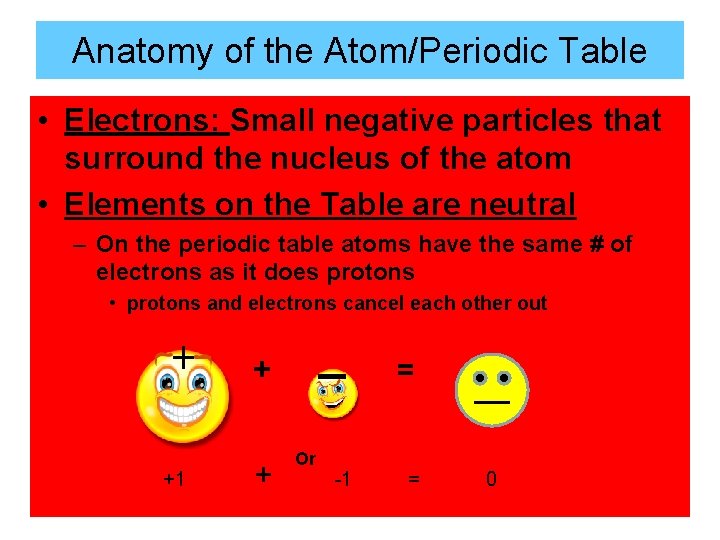
Anatomy of the Atom/Periodic Table • Electrons: Small negative particles that surround the nucleus of the atom • Elements on the Table are neutral – On the periodic table atoms have the same # of electrons as it does protons • protons and electrons cancel each other out + +1 _ + + Or -1 = = 0
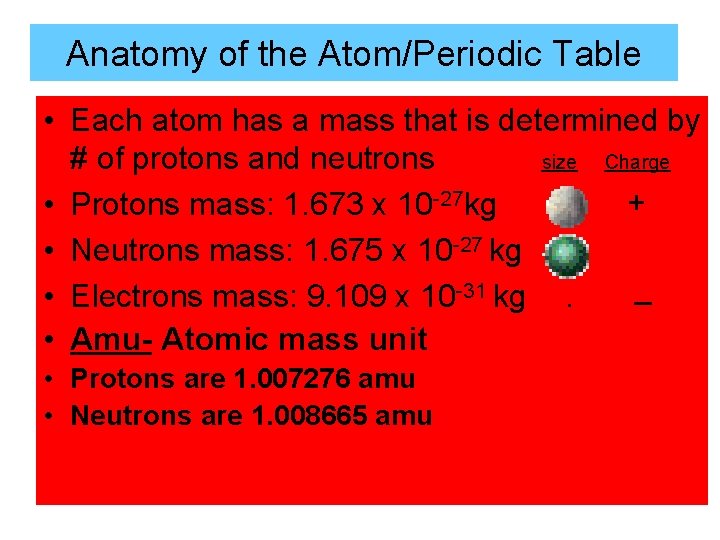
Anatomy of the Atom/Periodic Table • Each atom has a mass that is determined by # of protons and neutrons size Charge + • Protons mass: 1. 673 x 10 -27 kg • Neutrons mass: 1. 675 x 10 -27 kg _ • Electrons mass: 9. 109 x 10 -31 kg. • Amu- Atomic mass unit • Protons are 1. 007276 amu • Neutrons are 1. 008665 amu
Anatomy of the Atom/Periodic Table • Average atomic mass (Atomic Mass, Atomic Weight)-weighted average of the atomic masses of the different isotopes of an element. – Listed underneath each element on the table • Mass Number-The number of protons plus neutrons. This is NOT on the periodic Table. But it can be calculated.
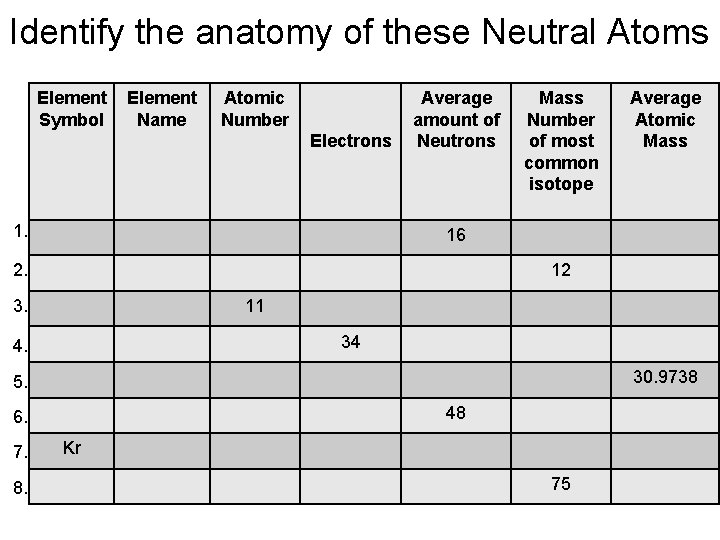
Identify the anatomy of these Neutral Atoms Element Symbol Element Name Atomic Number Electrons 1. Average amount of Neutrons Mass Number of most common isotope 16 2. 12 3. 11 34 4. 30. 9738 5. 48 6. 7. 8. Average Atomic Mass Kr 75
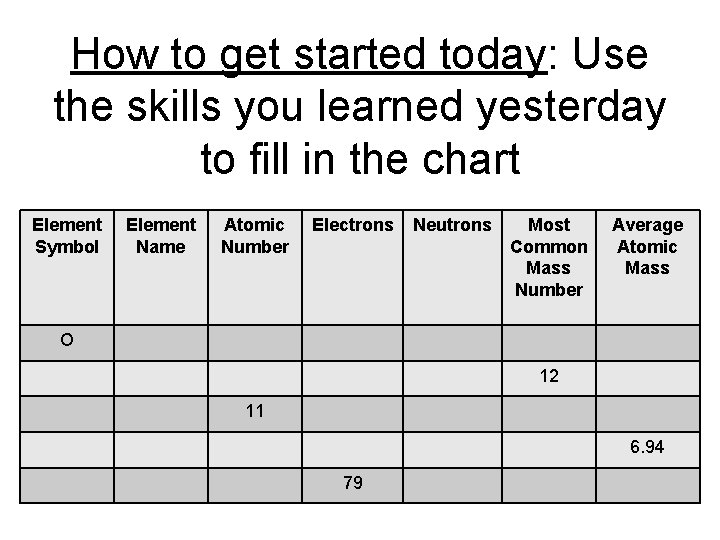
How to get started today: Use the skills you learned yesterday to fill in the chart Element Symbol Element Name Atomic Number Electrons Neutrons Most Common Mass Number Average Atomic Mass O 12 11 6. 94 79
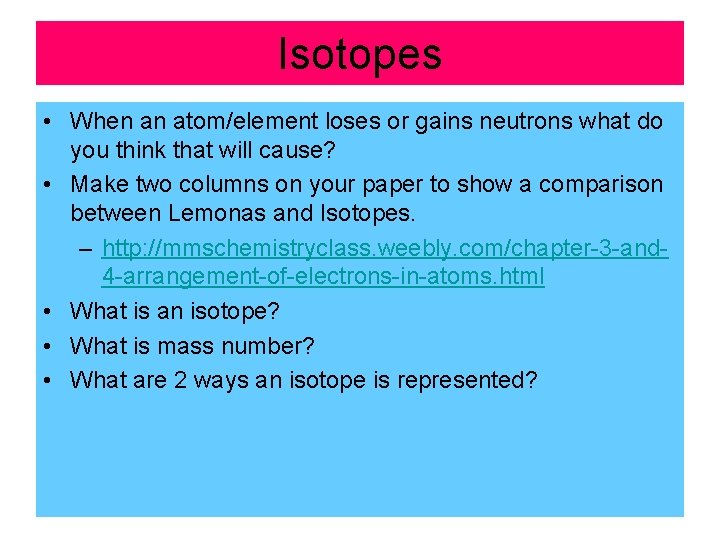
Isotopes • When an atom/element loses or gains neutrons what do you think that will cause? • Make two columns on your paper to show a comparison between Lemonas and Isotopes. – http: //mmschemistryclass. weebly. com/chapter-3 -and 4 -arrangement-of-electrons-in-atoms. html • What is an isotope? • What is mass number? • What are 2 ways an isotope is represented?
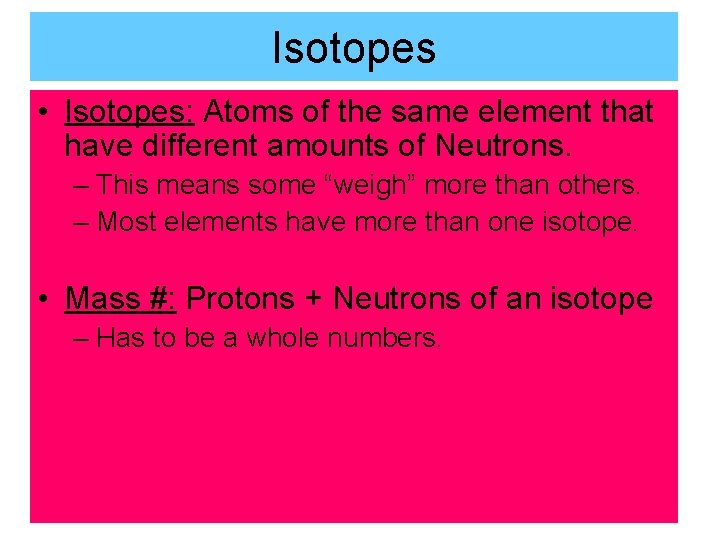
Isotopes • Isotopes: Atoms of the same element that have different amounts of Neutrons. – This means some “weigh” more than others. – Most elements have more than one isotope. • Mass #: Protons + Neutrons of an isotope – Has to be a whole numbers.
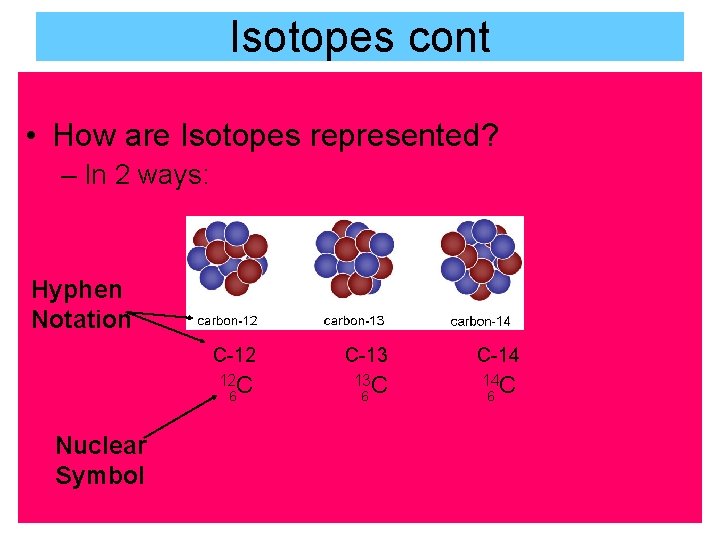
Isotopes cont • How are Isotopes represented? – In 2 ways: Hyphen Notation C-12 C-13 C-14 12 C 13 C 14 C 6 Nuclear Symbol 6 6
Isotopes
Anatomy of the Atom/Periodic Table Cont. • An atom/element may NOT change protons but it CAN lose or gain electrons and neutrons. • When an atom/element loses or gains electrons what do you think that will cause? • Atoms that gain or lose electrons are called Ions have a + or – charge. – Positive Charged atoms are called Cations • These atoms have lost electrons – Negative charged atoms are called Anions • These atoms have gained electrons
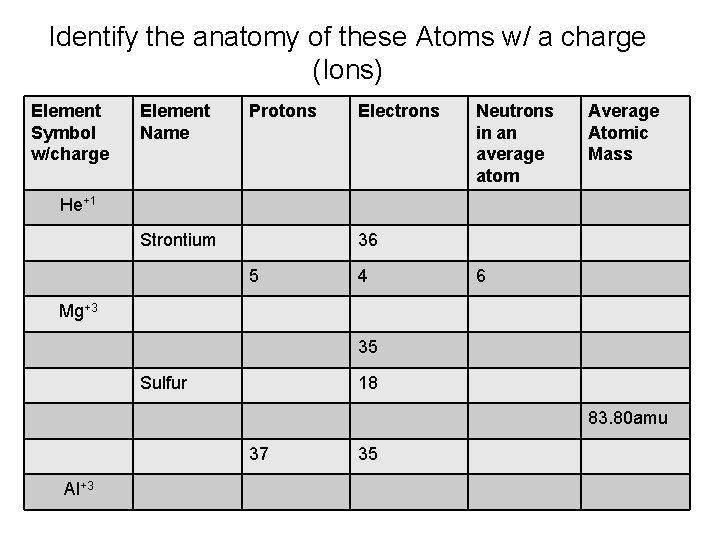
Identify the anatomy of these Atoms w/ a charge (Ions) Element Symbol w/charge Element Name Protons Electrons Neutrons in an average atom Average Atomic Mass He+1 Strontium 36 5 4 6 Mg+3 35 Sulfur 18 83. 80 amu 37 Al+3 35
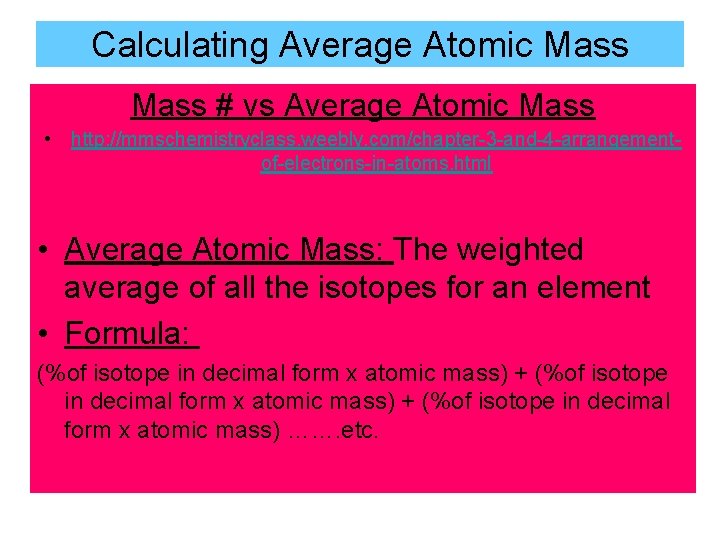
Calculating Average Atomic Mass # vs Average Atomic Mass • http: //mmschemistryclass. weebly. com/chapter-3 -and-4 -arrangementof-electrons-in-atoms. html • Average Atomic Mass: The weighted average of all the isotopes for an element • Formula: (%of isotope in decimal form x atomic mass) + (%of isotope in decimal form x atomic mass) ……. etc.
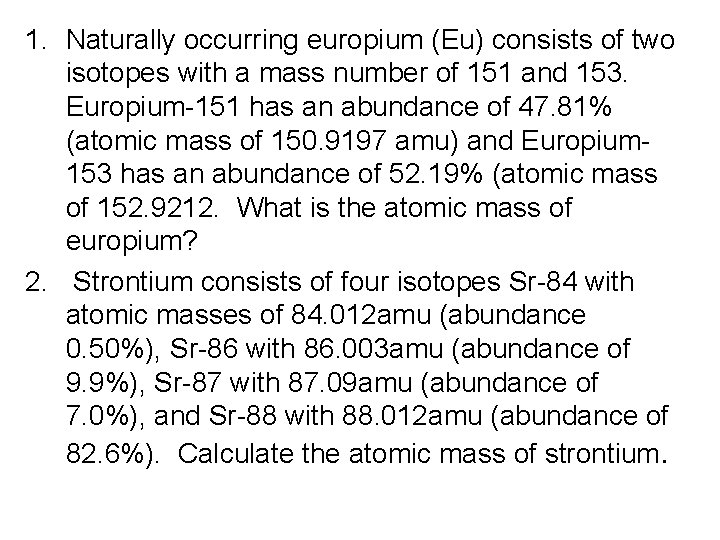
1. Naturally occurring europium (Eu) consists of two isotopes with a mass number of 151 and 153. Europium-151 has an abundance of 47. 81% (atomic mass of 150. 9197 amu) and Europium 153 has an abundance of 52. 19% (atomic mass of 152. 9212. What is the atomic mass of europium? 2. Strontium consists of four isotopes Sr-84 with atomic masses of 84. 012 amu (abundance 0. 50%), Sr-86 with 86. 003 amu (abundance of 9. 9%), Sr-87 with 87. 09 amu (abundance of 7. 0%), and Sr-88 with 88. 012 amu (abundance of 82. 6%). Calculate the atomic mass of strontium.
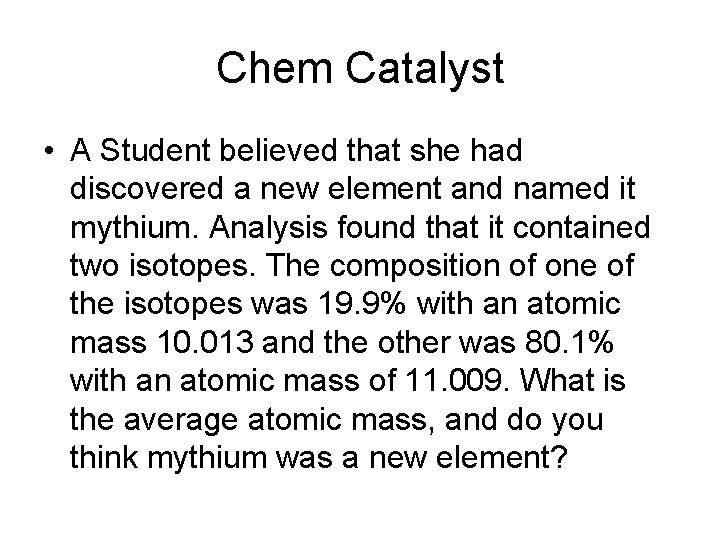
Chem Catalyst • A Student believed that she had discovered a new element and named it mythium. Analysis found that it contained two isotopes. The composition of one of the isotopes was 19. 9% with an atomic mass 10. 013 and the other was 80. 1% with an atomic mass of 11. 009. What is the average atomic mass, and do you think mythium was a new element?
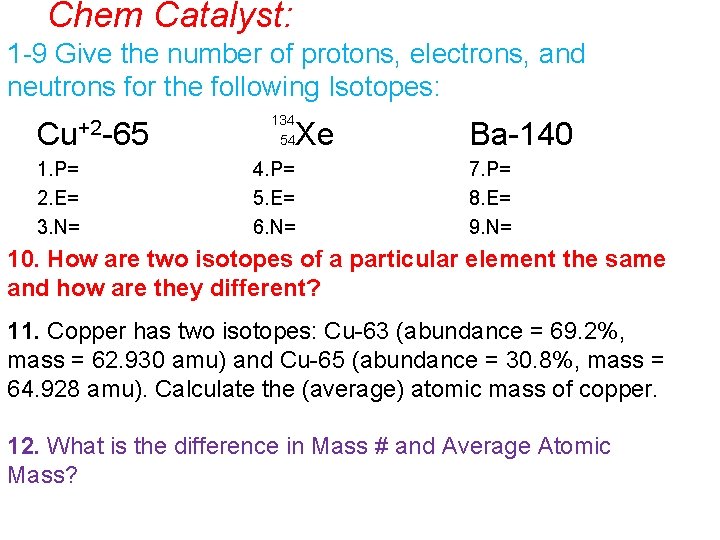
Chem Catalyst: 1 -9 Give the number of protons, electrons, and neutrons for the following Isotopes: Cu+2 -65 1. P= 2. E= 3. N= 134 54 Xe 4. P= 5. E= 6. N= Ba-140 7. P= 8. E= 9. N= 10. How are two isotopes of a particular element the same and how are they different? 11. Copper has two isotopes: Cu-63 (abundance = 69. 2%, mass = 62. 930 amu) and Cu-65 (abundance = 30. 8%, mass = 64. 928 amu). Calculate the (average) atomic mass of copper. 12. What is the difference in Mass # and Average Atomic Mass?
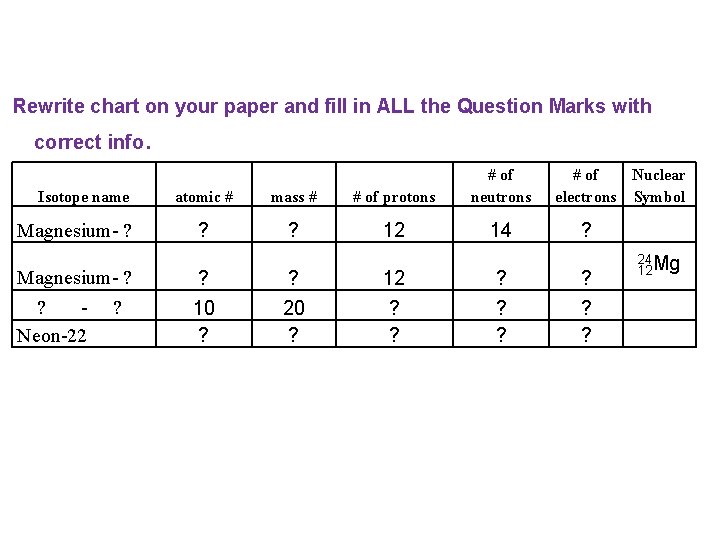
Rewrite chart on your paper and fill in ALL the Question Marks with correct info Isotope name Magnesium- ? ? Neon-22 ? . atomic # mass # # of protons # of neutrons ? ? 12 14 ? 10 ? ? 20 ? 12 ? ? ? # of Nuclear electrons Symbol ? ? 12 Mg 24
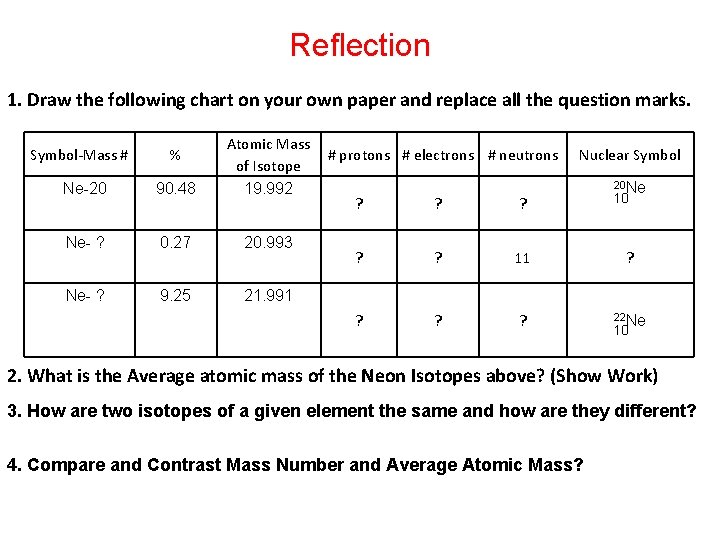
Reflection 1. Draw the following chart on your own paper and replace all the question marks. Ne-20 90. 48 Atomic Mass of Isotope 19. 992 Ne- ? 0. 27 20. 993 Ne- ? 9. 25 21. 991 Symbol-Mass # % # protons # electrons # neutrons ? ? ? 11 ? ? ? Nuclear Symbol 20 Ne 10 ? 22 Ne 10 2. What is the Average atomic mass of the Neon Isotopes above? (Show Work) 3. How are two isotopes of a given element the same and how are they different? 4. Compare and Contrast Mass Number and Average Atomic Mass?

How to get started today: Answer the following 1. Fe 2+ and Fe 3+ are different iron? a) b) c) d) Ions Isotopes Elements atoms 2. To change Li to Li+, you need to: a) b) c) d) add one electron remove one proton remove one electron add one neutron 3. Li+ and Cu 2+ are examples of: a) b) c) d) Anions Cations Isotopes molecules
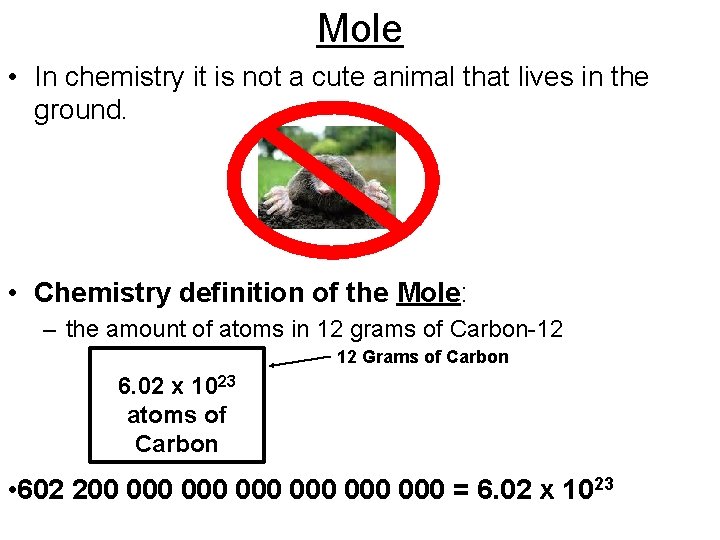
Mole • In chemistry it is not a cute animal that lives in the ground. • Chemistry definition of the Mole: – the amount of atoms in 12 grams of Carbon-12 12 Grams of Carbon 6. 02 x 1023 atoms of Carbon • 602 200 000 000 000 = 6. 02 x 1023
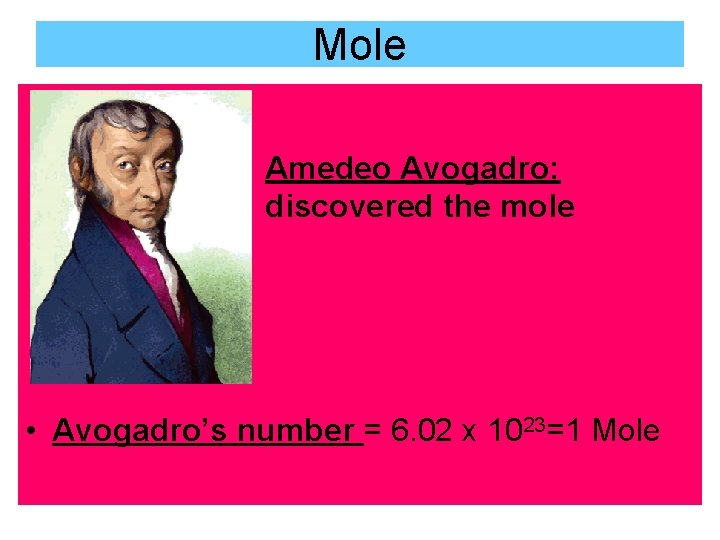
Mole Amedeo Avogadro: discovered the mole • Avogadro’s number = 6. 02 x 1023=1 Mole

• How many is a mole? If every person living on Earth (6 billion people) worked to count out one mole of oranges (or anything else), and if each person counted continually at a rate of one orange per second, it would take about 4 million years for all the oranges to be counted! • If we had a mole of sand it would cover the earth 7 times over! • If you had a mole of dollar bills, you could spend a million dollars every minute of your life and never spend it all! • Answer 1 -2 on the worksheet

Quiz 1. How was the mole determined? 2. What has more atoms, a mole of Hydrogen or a mole of Zinc? 3. How could molar mass be used as a conversion factor?
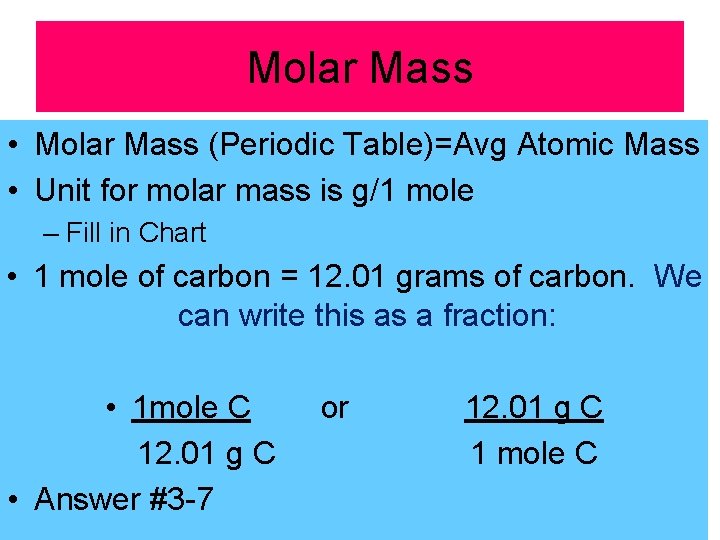
Molar Mass • Molar Mass (Periodic Table)=Avg Atomic Mass • Unit for molar mass is g/1 mole – Fill in Chart • 1 mole of carbon = 12. 01 grams of carbon. We can write this as a fraction: • 1 mole C 12. 01 g C • Answer #3 -7 or 12. 01 g C 1 mole C
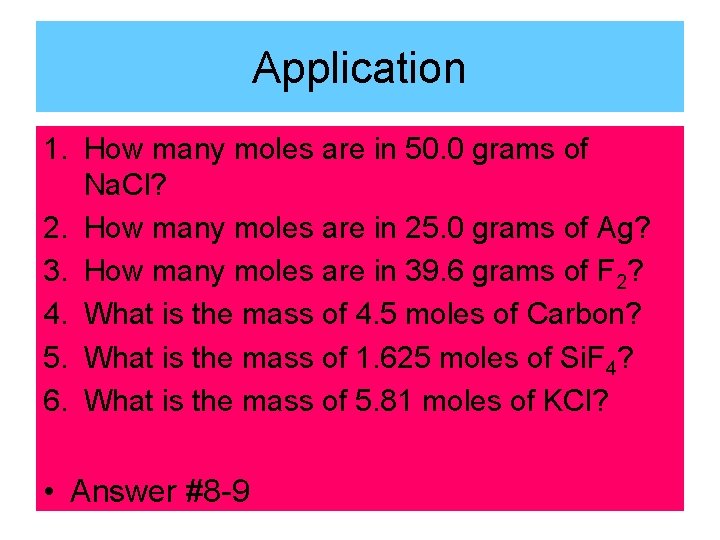
Application 1. How many moles are in 50. 0 grams of Na. Cl? 2. How many moles are in 25. 0 grams of Ag? 3. How many moles are in 39. 6 grams of F 2? 4. What is the mass of 4. 5 moles of Carbon? 5. What is the mass of 1. 625 moles of Si. F 4? 6. What is the mass of 5. 81 moles of KCl? • Answer #8 -9
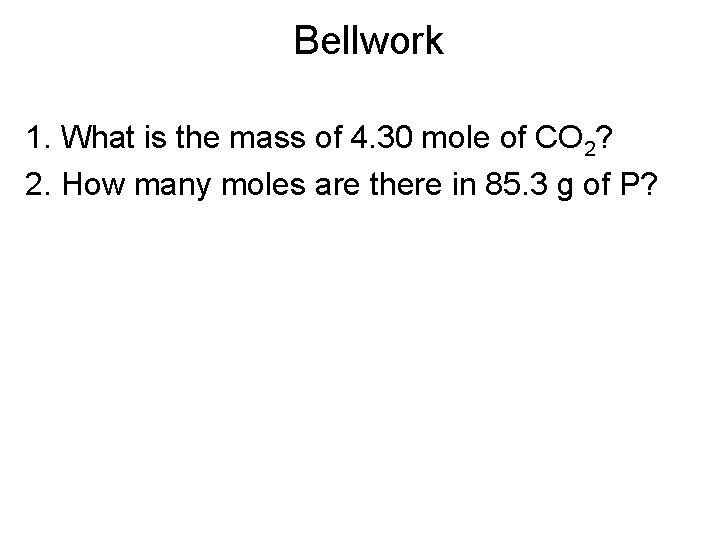
Bellwork 1. What is the mass of 4. 30 mole of CO 2? 2. How many moles are there in 85. 3 g of P?
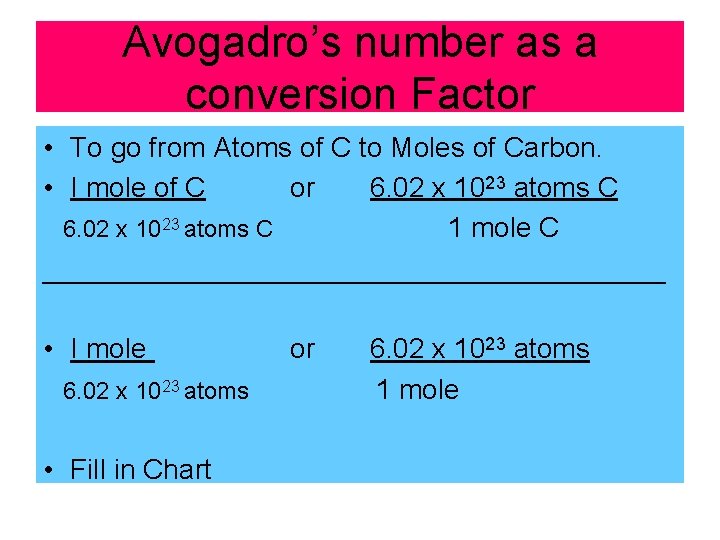
Avogadro’s number as a conversion Factor • To go from Atoms of C to Moles of Carbon. • I mole of C or 6. 02 x 1023 atoms C 1 mole C ____________________ • I mole 6. 02 x 1023 atoms • Fill in Chart or 6. 02 x 1023 atoms 1 mole

Application 1. How many atoms are in 4. 2 moles of Cu? 2. How many atoms are in 6. 5 moles of Zn? 3. How many moles are in 1. 5 x 1012 atoms H 2? 4. How many moles are in 2. 5 x 108 atoms Na? • Answer #10 -11
Chem Catalyst 1. What is the molar mass of CH 4? 2. How many molecules are in 5. 5 moles of silicon? 3. What conversion factor allows you to convert between Molecules and moles? 4. How many grams of H 2 O are needed to have a mole of water?

Quiz 1. How many moles are contained in 4. 5 x 1012 atoms of carbon? 2. What volume of Helium is in 6. 25 moles of Helium at 1 atm and 273 K?

Mole Island • You can not go directly from atoms to grams and vice versa. – To go from atoms to grams, first convert to moles, then grams. – To go from grams to atoms, first convert grams to mole, then moles to atoms.
Application 1. What is the mass of 4. 3 x 1018 atoms of C? 2. What is the mass of 2. 5 X 1012 atoms of Na. Br? 3. How many atoms are contained in 265. 0 grams of Ni? 4. How many atoms are contained in 25. 5 grams of Fe. O? 5. What amount of moles are found in 6. 71 X 1063 atoms of goop?
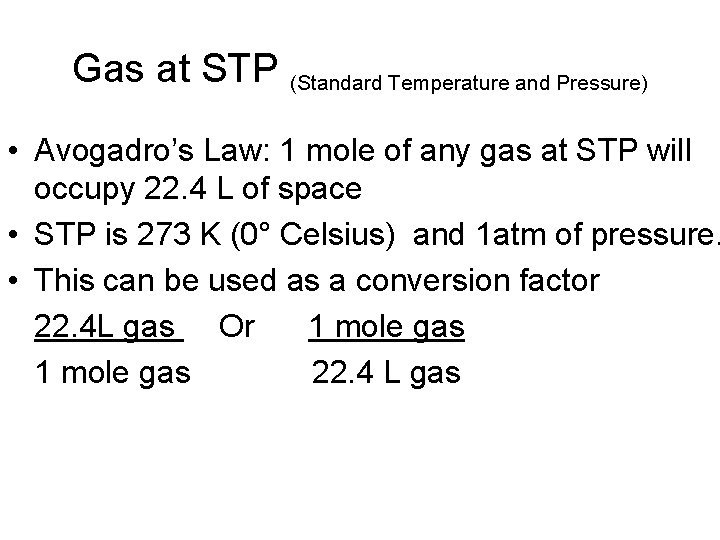
Gas at STP (Standard Temperature and Pressure) • Avogadro’s Law: 1 mole of any gas at STP will occupy 22. 4 L of space • STP is 273 K (0° Celsius) and 1 atm of pressure. • This can be used as a conversion factor 22. 4 L gas Or 1 mole gas 22. 4 L gas

Practice 1. Suppose you have a balloon inflated to 2. 00 L at STP. How many moles of gas are there? -. 0893 mol 2. What volume is occupied by 5. 00 moles of any gas at STP? - 112 L 3. What volume will 0. 75 moles of carbon CO 2 occupy at STP conditions? -16. 8 L 4. What volume is occupied by 12. 5 moles of nitrogen gas at STP? -280 L 5. What volume is occupied by 100. 0 grams of chlorine gas (Cl 2) at STP? -31. 58 L 6. How many liters will 48. 6 grams of CO 2 occupy at 1 atm and 273 K? -24. 7 L 7. What is the mass in grams of 17. 0 liters of methane gas (CH 4) at STP? -12. 2 g

How to get started today: 1. Write the hyphen notation for the element that contains 18 protons and 22 neutrons. 2. Write the Nuclear Symbol for the element that has a mass number of 76, an atomic number of 33, and 31 electrons. 3. If you have 91. 2 L of CO(carbon monoxide) then how many molecules are present?

Bellwork • Grab a power point page from the table and make sure that info is in your notes. • People who were here may help those who weren’t • You may talk today but only if it is about this stuff
Molar Conversion Quiz 1. What is the mass of 4. 5 x 1012 molecules of KOH? 2. What mass of NH 3 (ammonia) gas is in 2. 5 L of ammonia gas at STP? 3. How many pieces of sushi are in 5. 2 moles of sushi? 4. If you have 62. 1 g of Fe then how many moles do you have?
Photoelectric Effect(Early 1900’s) • Photoelectric effect-Heated metal absorbs energy and causes electrons to get excited. • Photon of energy-Particle of light that is carrying enough energy to excite an electron – It takes a quantum of energy to excite an electron - Photon Quantum of energy Woohoo!!
- Slides: 44