Atoms Smallest part of all matter Made of


















- Slides: 34

Atoms Smallest part of all matter Made of smaller particles Dalton Rutherford Thompson Chadwick Bohr

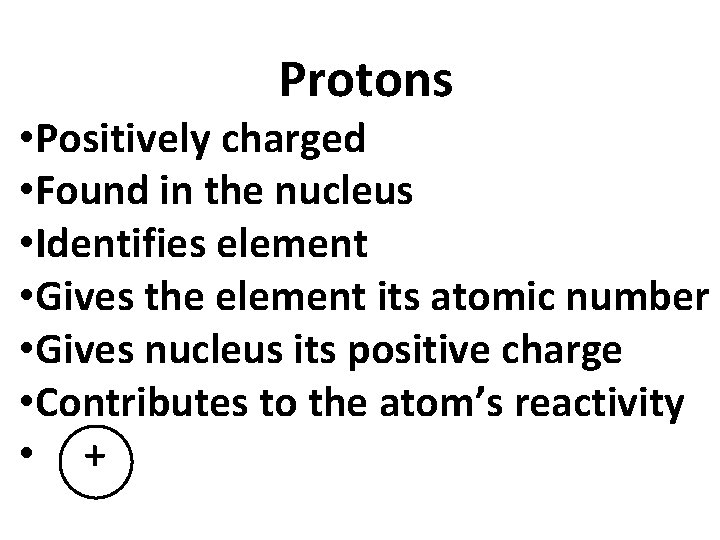
Protons • Positively charged • Found in the nucleus • Identifies element • Gives the element its atomic number • Gives nucleus its positive charge • Contributes to the atom’s reactivity • +

NEUTRONS §Neutrally charged-no charge §Found in the nucleus §Contributes to the mass of the atom § ‘n’

ELECTRONS o. Negatively charged o. Found on the energy shells o. Determines the reactivity of the atom o. Has very small mass-not normally considered when calculating mass of atom. o e

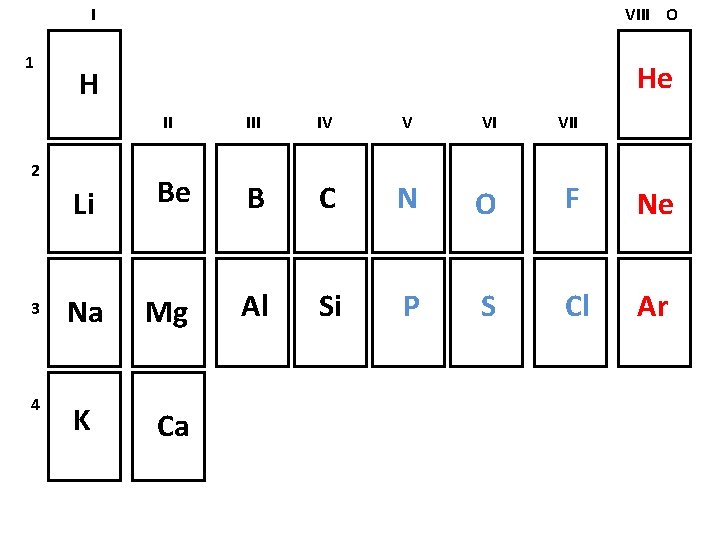
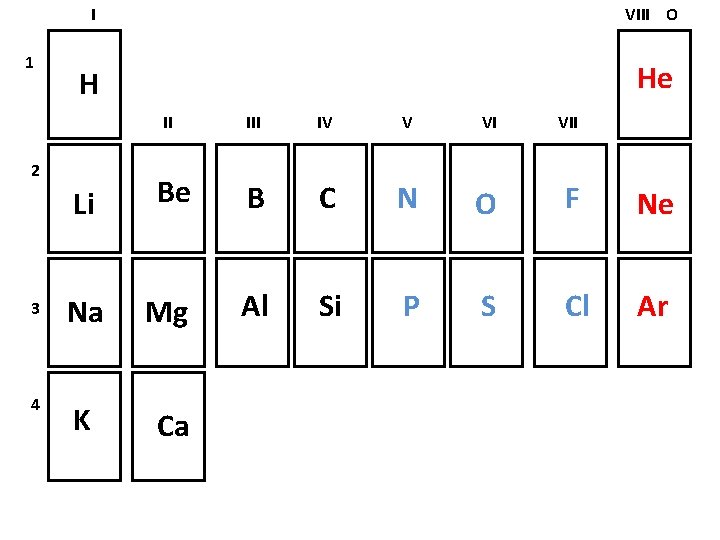
1 I VIII O H He II 2 III IV V VI VII Li Be B C N O F Ne 3 Na Mg Al Si P S Cl Ar 4 K Ca

Elements can be classified as Metals: found on the left side of the periodic table less than 4 valence electrons solids at room temperature Nonmetals: found on the right side of the periodic table more than 4 valence electrons gases at room temperature Noble gases: found in the last column unreactive under normal situations gases at room temperature

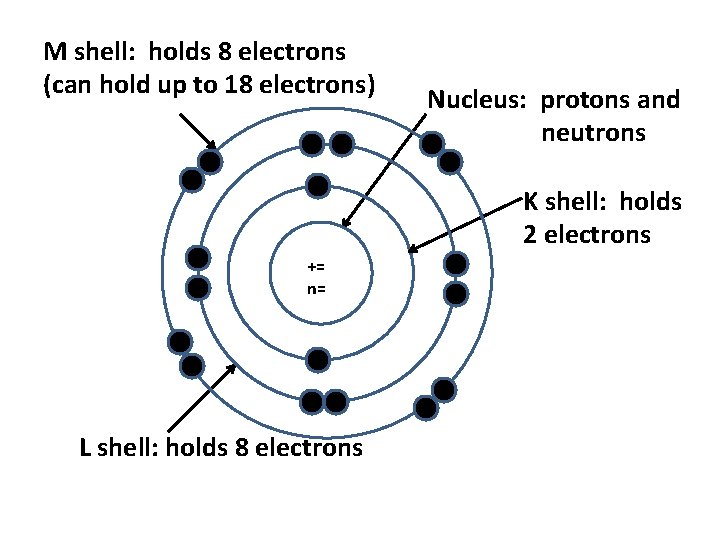
M shell: holds 8 electrons (can hold up to 18 electrons) Nucleus: protons and neutrons K shell: holds 2 electrons += n= L shell: holds 8 electrons

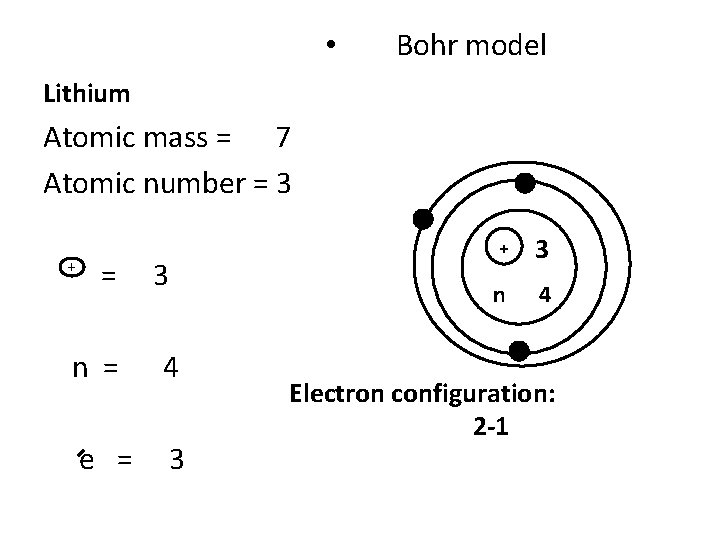
• Bohr model Lithium Atomic mass = 7 Atomic number = 3 n = 4 e = 3 + + 3 n 4 Electron configuration: 2 -1

Electron dot diagram is a way of representing only the outer most shell. This shell is called the valence shell, is often unstable, and will be the shell involved in bonding. Li F

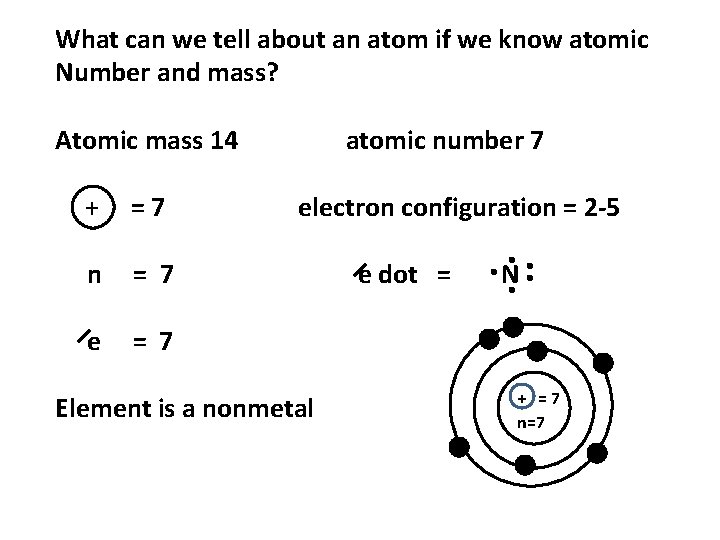
What can we tell about an atom if we know atomic Number and mass? Atomic mass 14 + =7 n = 7 e = 7 atomic number 7 electron configuration = 2 -5 Element is a nonmetal e dot = N + =7 n=7

Elements can be classified as Metals: found on the left side of the periodic table less than 4 valence electrons solids at room temperature Nonmetals: found on the right side of the periodic table more than 4 valence electrons gases at room temperature Noble gases: found in the last column unreactive under normal situations gases at room temperature

Periodic table arranges elements according to atomic number increases by one as we read from left to right atomic number will determine reactivity

Remember, valence means having to do with the last shell. Atoms like to use the least amount of energy. that means, they like to have their electrons paired and to have the valence shell stable with 8 electrons. There are only 6 elements that naturally exist in this stable state. They are ……. Noble gases

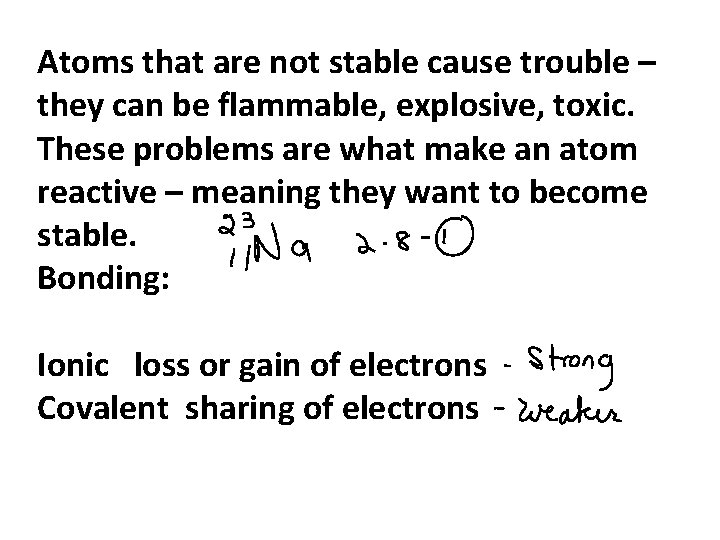
Atoms that are not stable cause trouble – they can be flammable, explosive, toxic. These problems are what make an atom reactive – meaning they want to become stable. Bonding: Ionic loss or gain of electrons Covalent sharing of electrons

IONIC BONDING: OCCURS BETWEEN- METALS: LESS THAN 4 VALENCE ELECTRONS AND NONMETALS: MORE THAN 4 VALENCE ELECTRONS ***REMEMBER: ATOMS WANT TO USE LITTLE E!! METALS WILL LOSE ELECTRONS NONMETALS WILL GAIN THOSE ELECTRONS LOST

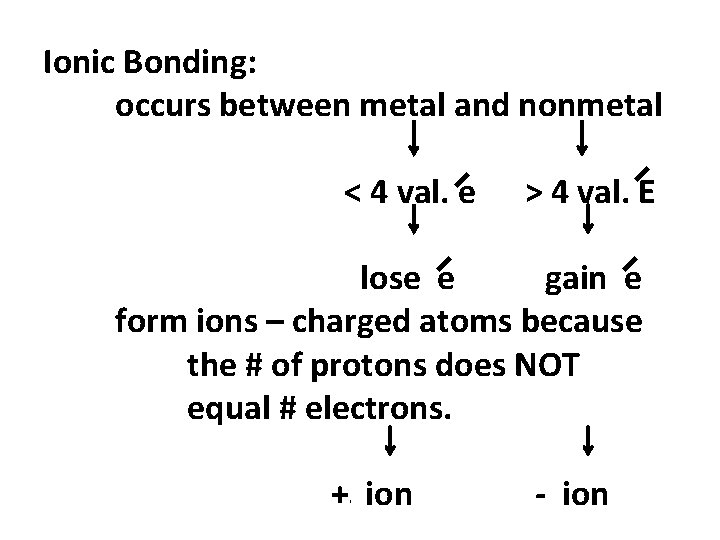
Ionic Bonding: occurs between metal and nonmetal < 4 val. e > 4 val. E lose e gain e form ions – charged atoms because the # of protons does NOT equal # electrons. + ion - ion

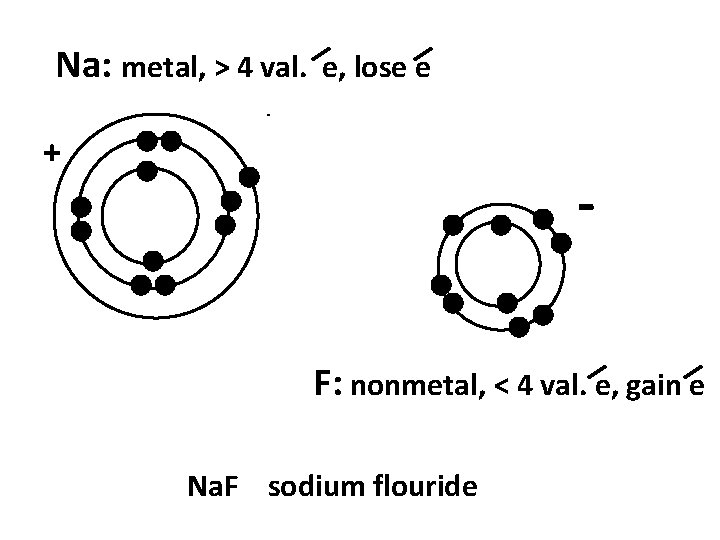
Na: metal, > 4 val. e, lose e + F: nonmetal, < 4 val. e, gain e Na. F sodium flouride

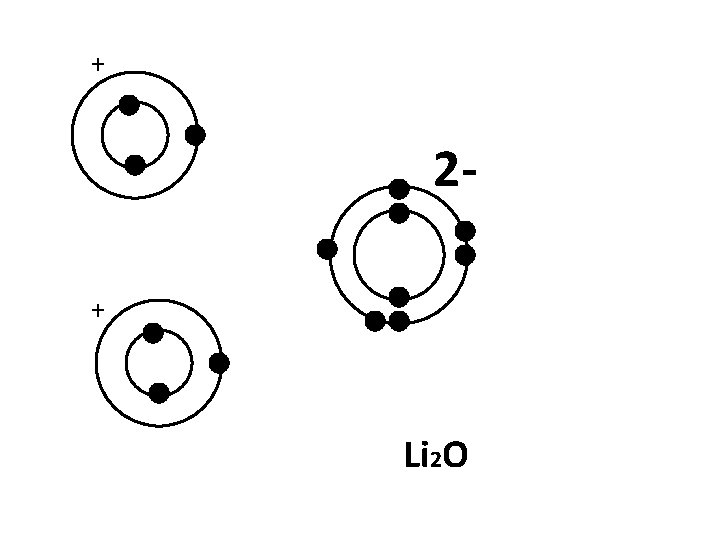
+ 2+ Li 2 O

Bonding between atoms is a result of: a. atoms need to be stable b. atoms need to exist in a lower energy state c. attraction of positive nucleus and negative electrons d. size of the atom’s radius

Atomic radius=distance between nucleus and valence shell. the + nucleus will hold the – electrons on the atom by means of attractive forces. As atomic radius gets larger, attractive forces get smaller – electrons lost easily. That’s good for a metal. as atomic radius gets smaller, attractive forces get stronger. That’s good for a nonmetal.

1 I VIII O H He II 2 III IV V VI VII Li Be B C N O F Ne 3 Na Mg Al Si P S Cl Ar 4 K Ca

COVALENT BONDING ØSHARING OF ELECTRONS ØATOMS REMAIN NEUTRAL ØOCCURS BETWEEN: v 2 NONMETALS v. A NONMETAL AND A METALLOID v. HYDROGEN AND ALL NONMETALS ØPRODUCES WEAK BONDS ØPRODUCES COMPOUNDS THAT HAVE LOW MELTING POINTS ØALL ORGANIC COMPOUNDS ARE COVALENT

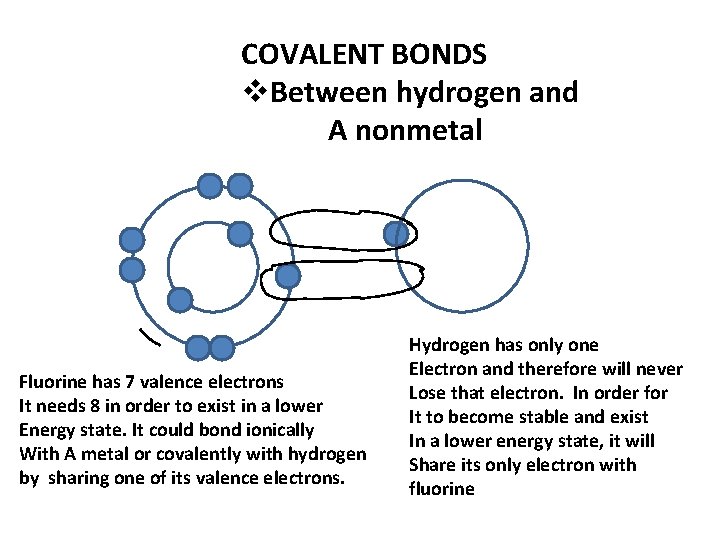
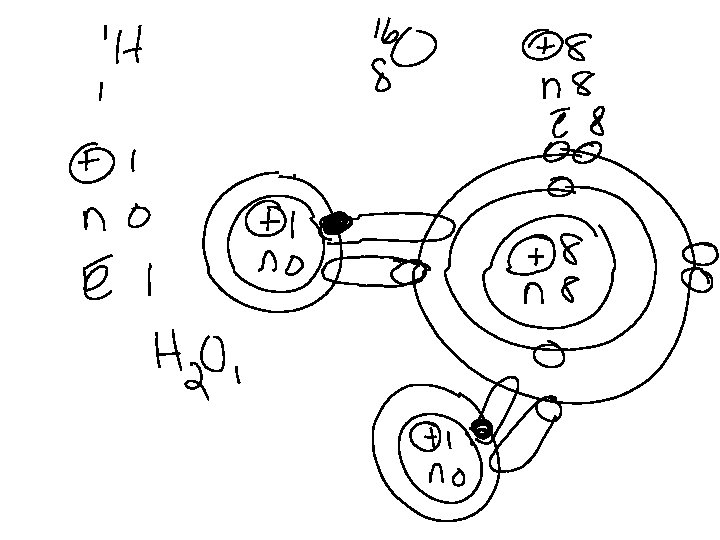
COVALENT BONDS v. Between hydrogen and A nonmetal Fluorine has 7 valence electrons It needs 8 in order to exist in a lower Energy state. It could bond ionically With A metal or covalently with hydrogen by sharing one of its valence electrons. Hydrogen has only one Electron and therefore will never Lose that electron. In order for It to become stable and exist In a lower energy state, it will Share its only electron with fluorine

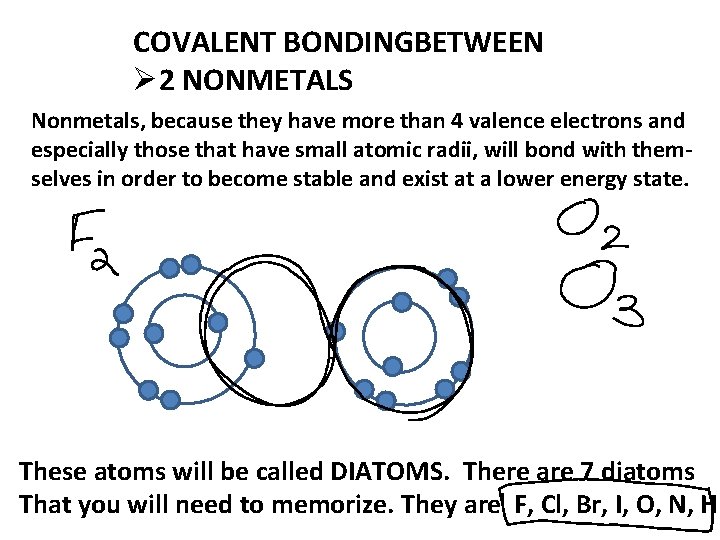
COVALENT BONDINGBETWEEN Ø 2 NONMETALS Nonmetals, because they have more than 4 valence electrons and especially those that have small atomic radii, will bond with themselves in order to become stable and exist at a lower energy state. These atoms will be called DIATOMS. There are 7 diatoms That you will need to memorize. They are F, Cl, Br, I, O, N, H.

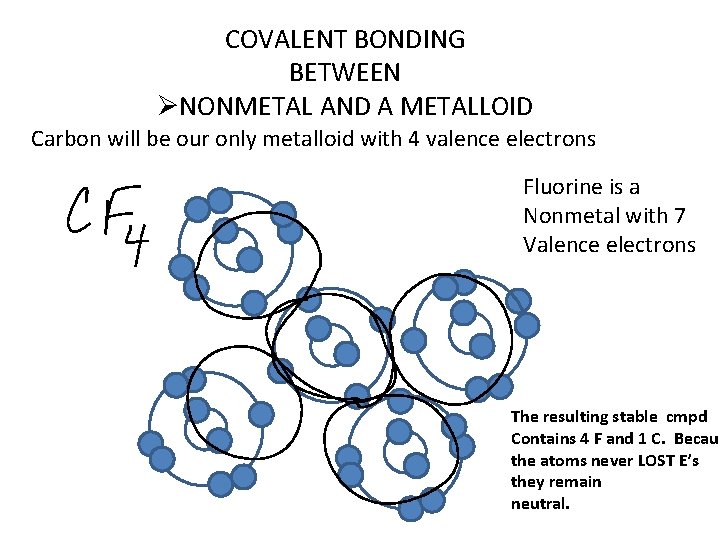
COVALENT BONDING BETWEEN ØNONMETAL AND A METALLOID Carbon will be our only metalloid with 4 valence electrons Fluorine is a Nonmetal with 7 Valence electrons The resulting stable cmpd Contains 4 F and 1 C. Becaus the atoms never LOST E’s they remain neutral.

Comparison of bonds • Ionic Bonding: • occurs between metal and nonmetal • lose e gain e • form ions – charged atoms • COVALENT BONDING Ø SHARING OF ELECTRONS Ø ATOMS REMAIN NEUTRAL Ø OCCURS BETWEEN: v 2 NONMETALS v A NONMETAL AND A METALLOID v HYDROGEN AND ALL NONMETALS





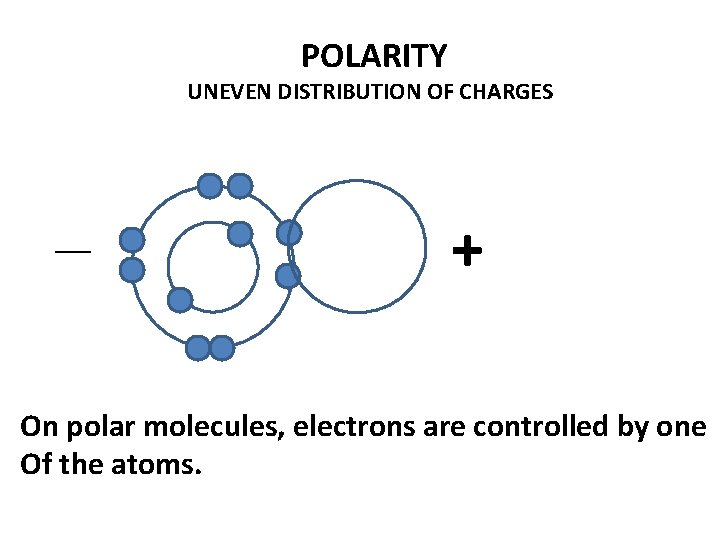
POLARITY UNEVEN DISTRIBUTION OF CHARGES __ + On polar molecules, electrons are controlled by one Of the atoms.


