Atoms Protons positive charged particle found in nucleus





![� p. H scale �Ranges from 0 to 14 and indicates the [H+]. �Acids � p. H scale �Ranges from 0 to 14 and indicates the [H+]. �Acids](https://slidetodoc.com/presentation_image_h2/694a4435944d45734bae700b21cc250f/image-15.jpg)
- Slides: 17


� Atoms �Protons- positive charged particle found in nucleus (# of protons determines element) �Neutron- neutral charged particle found in nucleus �Electrons- negatively charged particle found in electron cloud � Elements- smallest form of matter, made up of the same atoms � 92 naturally occurring elements � 6 are basic to life and make up 95% of the body weight of organisms- CHNOPS

� Isotopes �Atom of the same element with a different mass number �Carbon has 3 common isotopes- Carbon-12, Carbon 13, and Carbon 14 Because of the unstable ratio of protons to neutrons in Carbon- 14, it is radioactive (releases energy) and decays into nitrogen-14.


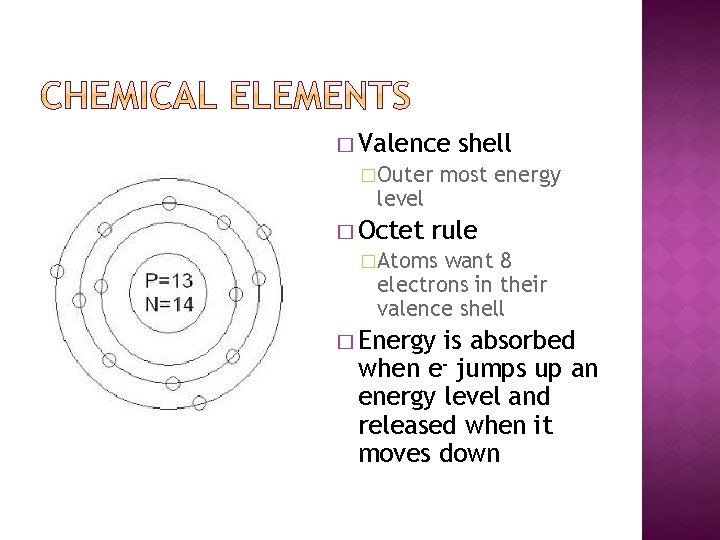
� Valence �Outer level � Octet shell most energy rule �Atoms want 8 electrons in their valence shell � Energy is absorbed when e- jumps up an energy level and released when it moves down

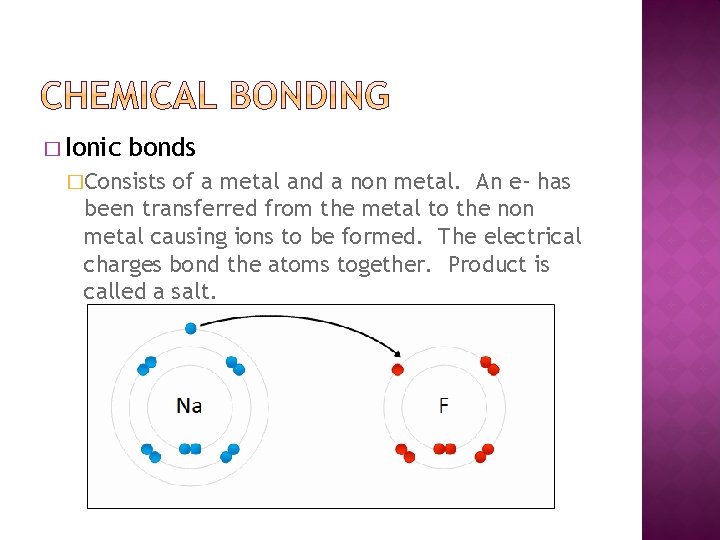
� Ionic bonds �Consists of a metal and a non metal. An e- has been transferred from the metal to the non metal causing ions to be formed. The electrical charges bond the atoms together. Product is called a salt.

� Covalent � 2 Bonding or more atoms share electrons to complete the octet rule. Can have single, double or triple bonds.

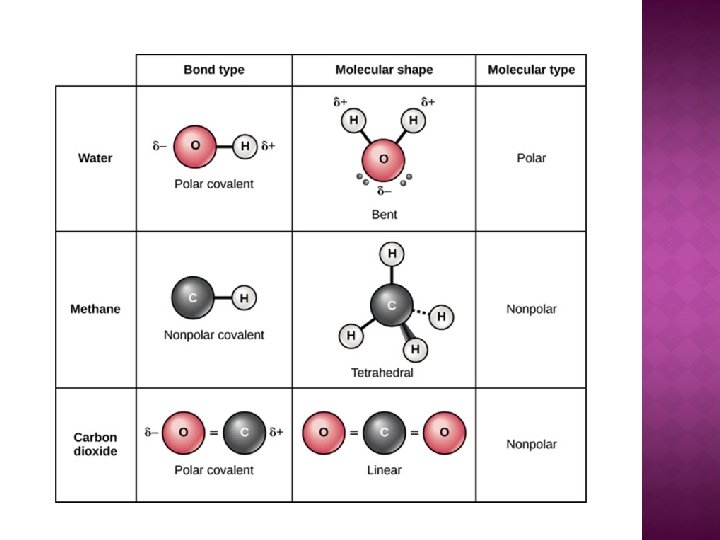
� Polar and Non-polar Covalent Bonds �When atoms have differences in electronegativity, they create an unequal distribution of e- within the molecule. This gives the molecule a partial positive end a partial negative end: a polar molecule. �When there is an equal distribution of e-, the molecule is non polar.


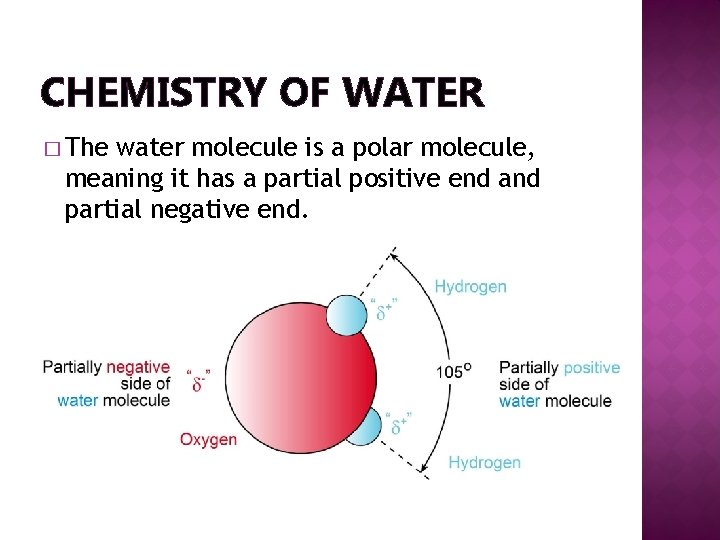
CHEMISTRY OF WATER � The water molecule is a polar molecule, meaning it has a partial positive end and partial negative end.

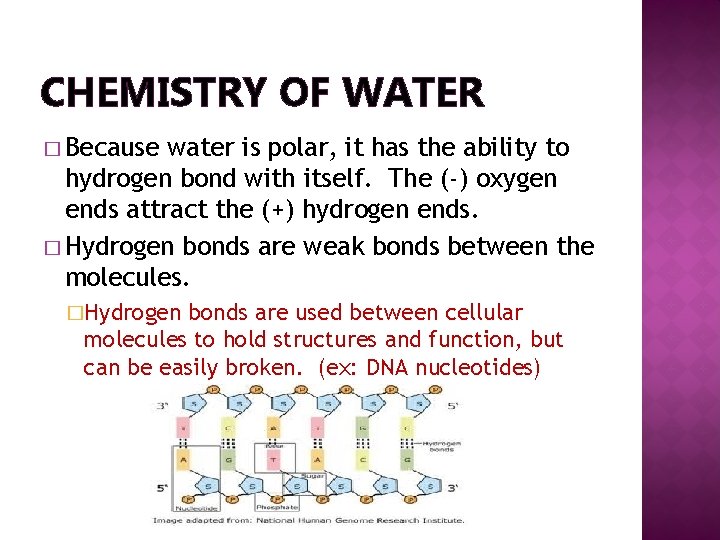
CHEMISTRY OF WATER � Because water is polar, it has the ability to hydrogen bond with itself. The (-) oxygen ends attract the (+) hydrogen ends. � Hydrogen bonds are weak bonds between the molecules. �Hydrogen bonds are used between cellular molecules to hold structures and function, but can be easily broken. (ex: DNA nucleotides)

PROPERTIES OF WATER � High Heat Capacity- requires a lot of energy to raise the temperature of 1 g of water by 1◦ C. (1 calorie or 4. 18 Joules) �This is important to living things because it helps maintain a steady body temperature. � High Heat of Evaporation- 540 calories are required to evaporate 1 g of water. �This helps animals release excess body heat (sweat)

� Water is said to be the universal solvent. �Because of it’s polarity, water can dissolve a large number of solutes, like salt. �Since water molecules can adhere to one another and other substances, it can transport nutrients and waste throughout the body. �Hydrophobic substances- “water fearing” These don’t attract to water �Hydrophilic substances- “water loving” these substances do attract to water. This is important for the phospholipids in our plasma membrane.

� Acids produce Hydrogen Ions (H+) � Bases produces Hydroxide Ions (OH-) � The strength or weakness of an acid or base depends on how well it dissociates in water. �Acids have a high [H+] �Bases have a high [OH-]
![p H scale Ranges from 0 to 14 and indicates the H Acids � p. H scale �Ranges from 0 to 14 and indicates the [H+]. �Acids](https://slidetodoc.com/presentation_image_h2/694a4435944d45734bae700b21cc250f/image-15.jpg)
� p. H scale �Ranges from 0 to 14 and indicates the [H+]. �Acids range from 0 to <7. �Bases range from >7 to 14. �Neutral solutions have a p. H of 7 and [H+]=[OH-]

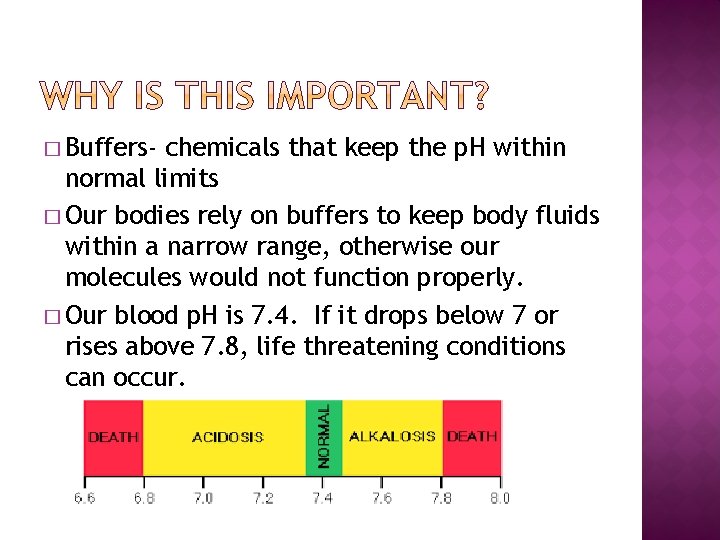
� Buffers- chemicals that keep the p. H within normal limits � Our bodies rely on buffers to keep body fluids within a narrow range, otherwise our molecules would not function properly. � Our blood p. H is 7. 4. If it drops below 7 or rises above 7. 8, life threatening conditions can occur.

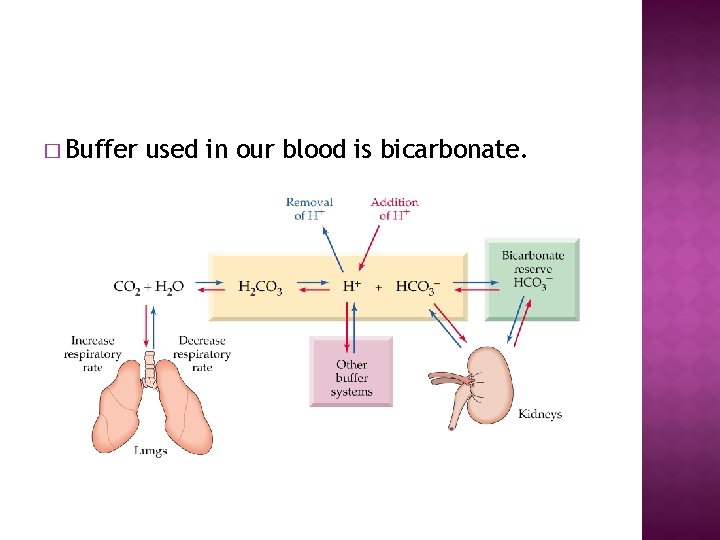
� Buffer used in our blood is bicarbonate.