Atoms Presented by Kesler Science Essential Questions 1

Atoms Presented by Kesler Science
Essential Questions: 1. What is the basic structure of atoms? 2. How is an atom’s mass calculated? 3. Which subatomic particles are electrically charged? .
Essential Questions: 4. Where are three main subatomic particles located? 5. How do protons determine an atom’s identity?
Atoms What is an atom? • The basic building blocks of ordinary matter • The basic unit of a chemical element. (ex: gold, oxygen, mercury) • Consists of 3 basic parts - protons, neutrons, electrons (also called subatomic particles) © Kesler. Science. com
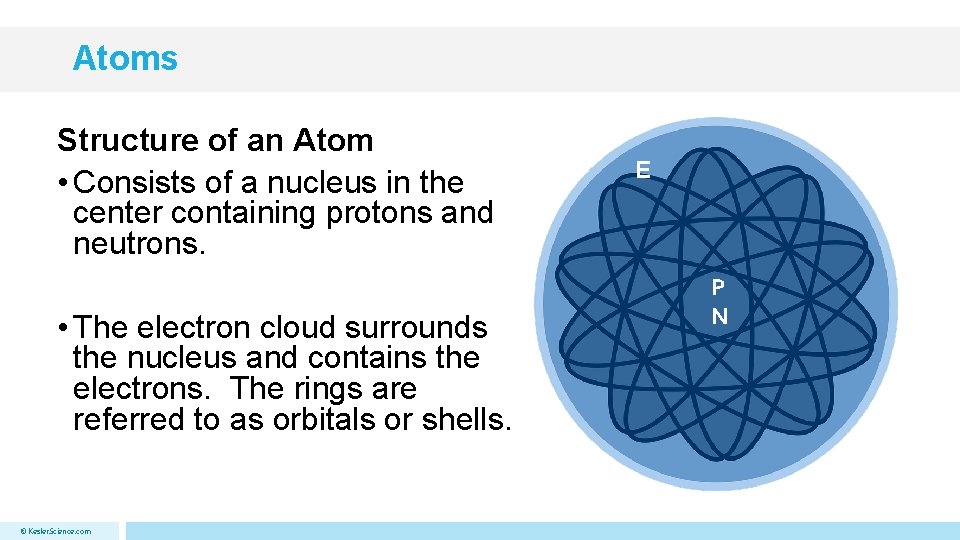
Atoms Structure of an Atom • Consists of a nucleus in the center containing protons and neutrons. • The electron cloud surrounds the nucleus and contains the electrons. The rings are referred to as orbitals or shells. © Kesler. Science. com E P N
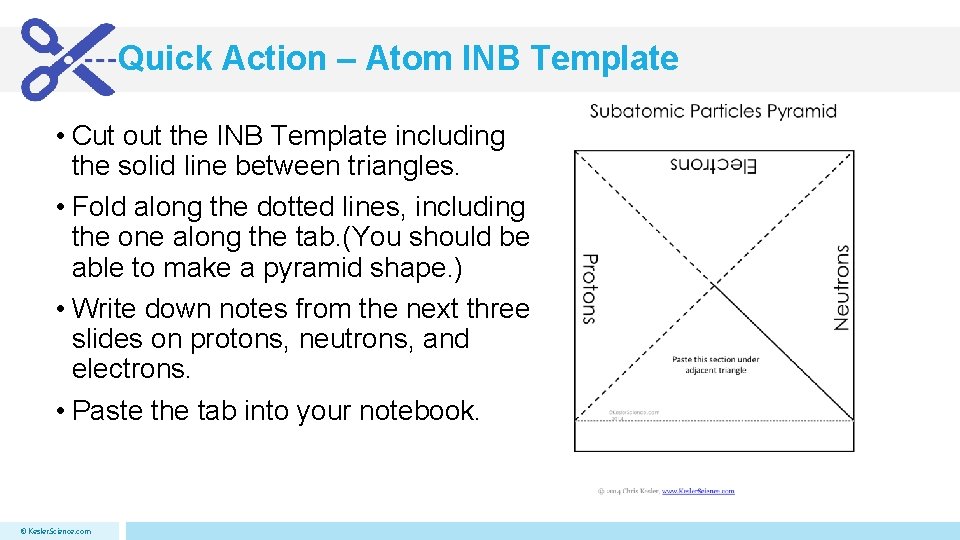
Quick Action – Atom INB Template • Cut out the INB Template including the solid line between triangles. • Fold along the dotted lines, including the one along the tab. (You should be able to make a pyramid shape. ) • Write down notes from the next three slides on protons, neutrons, and electrons. • Paste the tab into your notebook. © Kesler. Science. com

Quick Action – Atom INB Template • Cut out the INB Template including the solid line between triangles. • Fold along the dotted lines, including the one along the tab. (You should be able to make a pyramid shape. ) • Write down notes from the next three slides on protons, neutrons, and electrons. • Paste the tab into your notebook. © Kesler. Science. com Subatomic Particles Pyramid
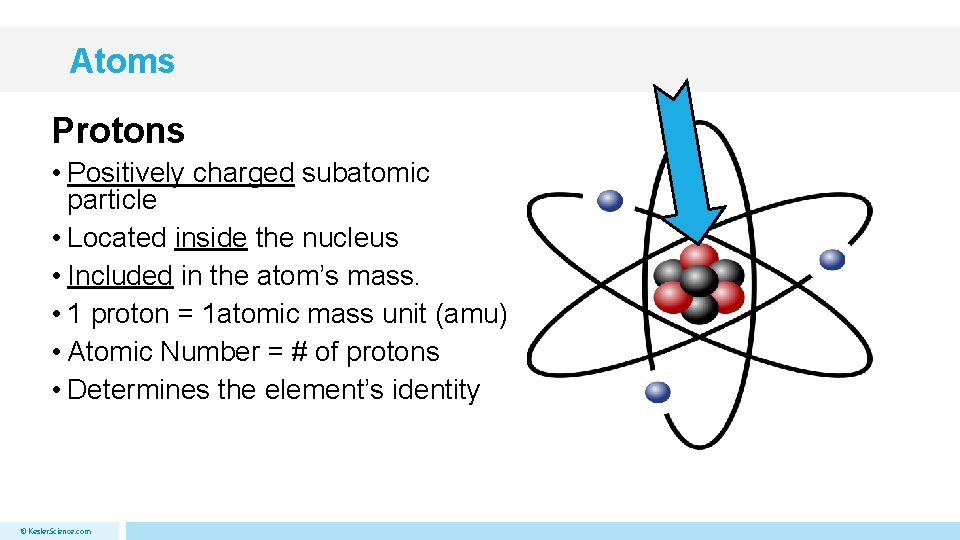
Atoms Protons • Positively charged subatomic particle • Located inside the nucleus • Included in the atom’s mass. • 1 proton = 1 atomic mass unit (amu) • Atomic Number = # of protons • Determines the element’s identity © Kesler. Science. com
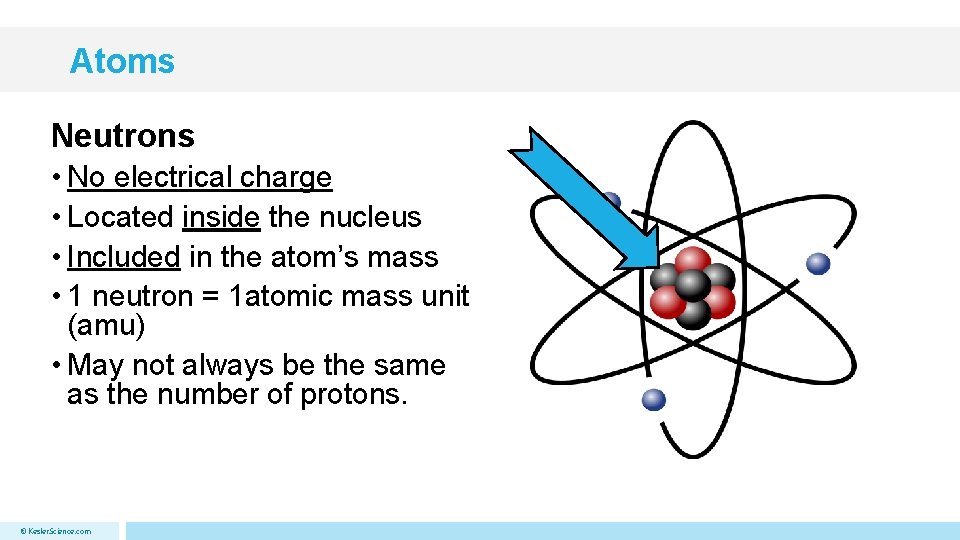
Atoms Neutrons • No electrical charge • Located inside the nucleus • Included in the atom’s mass • 1 neutron = 1 atomic mass unit (amu) • May not always be the same as the number of protons. © Kesler. Science. com
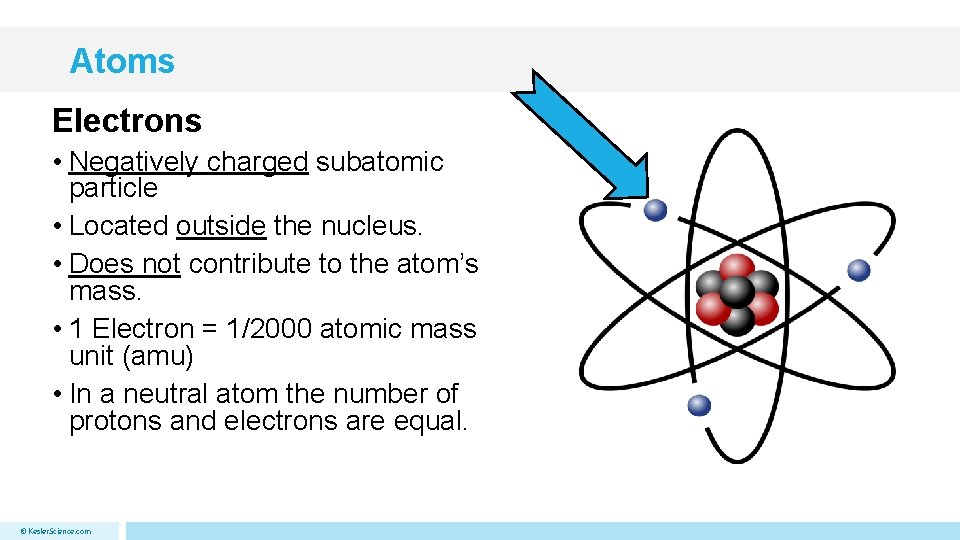
Atoms Electrons • Negatively charged subatomic particle • Located outside the nucleus. • Does not contribute to the atom’s mass. • 1 Electron = 1/2000 atomic mass unit (amu) • In a neutral atom the number of protons and electrons are equal. © Kesler. Science. com
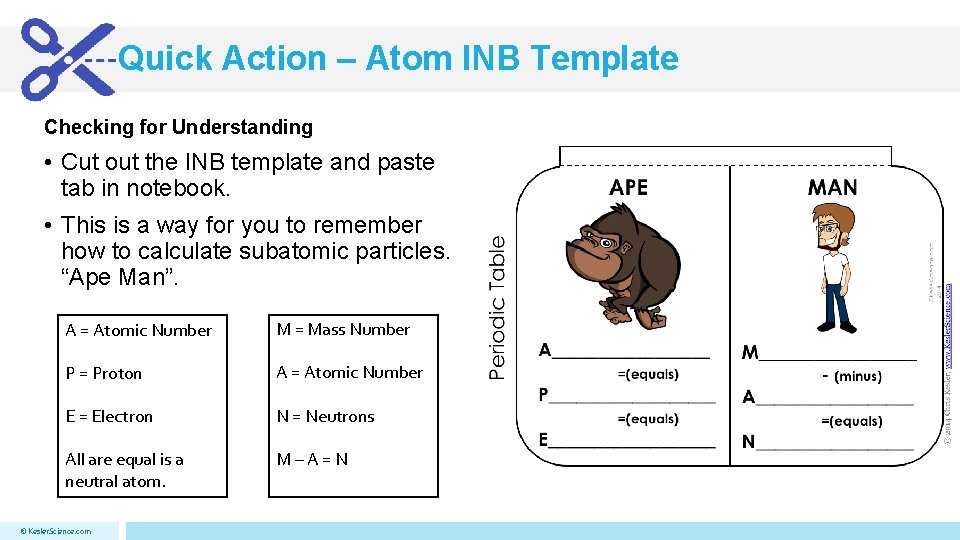
Quick Action – Atom INB Template Checking for Understanding • Cut out the INB template and paste tab in notebook. • This is a way for you to remember how to calculate subatomic particles. “Ape Man”. A = Atomic Number M = Mass Number P = Proton A = Atomic Number E = Electron N = Neutrons All are equal is a neutral atom. M–A=N © Kesler. Science. com
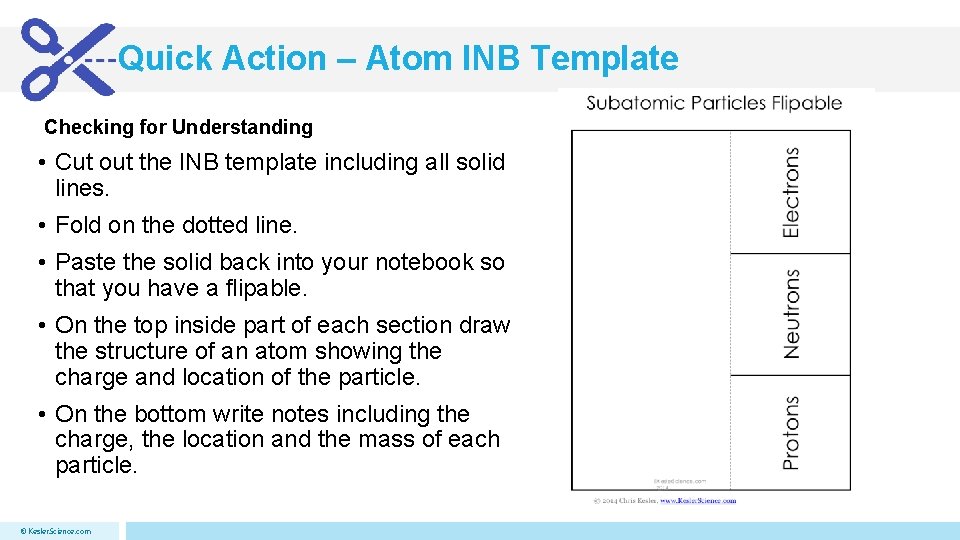
Quick Action – Atom INB Template Checking for Understanding • Cut out the INB template including all solid lines. • Fold on the dotted line. • Paste the solid back into your notebook so that you have a flipable. • On the top inside part of each section draw the structure of an atom showing the charge and location of the particle. • On the bottom write notes including the charge, the location and the mass of each particle. © Kesler. Science. com
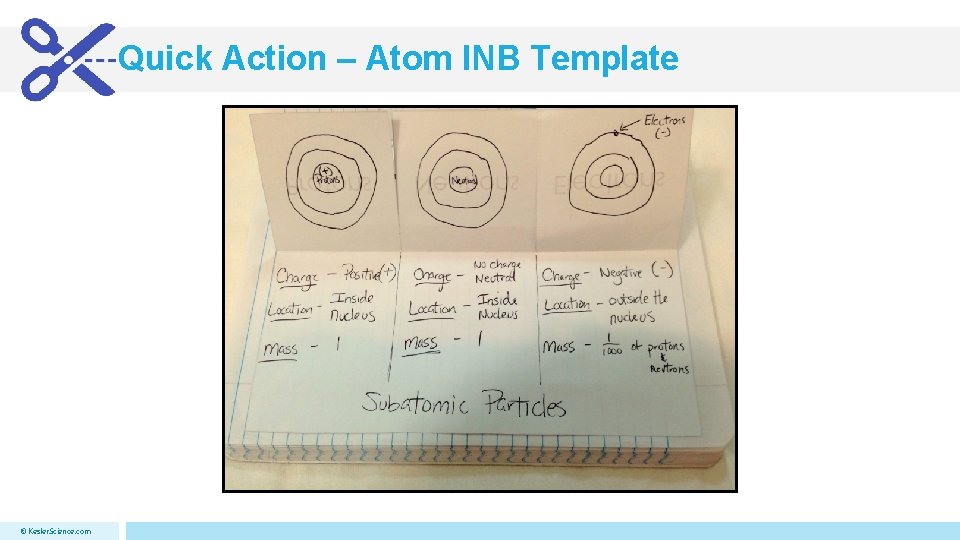
Quick Action – Atom INB Template © Kesler. Science. com
Atoms Periodic Table • This table shows all the different atoms there are on Earth. • It’s called the Periodic Table of Elements. • Remember, an atom is the basic unit of a chemical element. Let’s take a closer look at one atom(element). © Kesler. Science. com
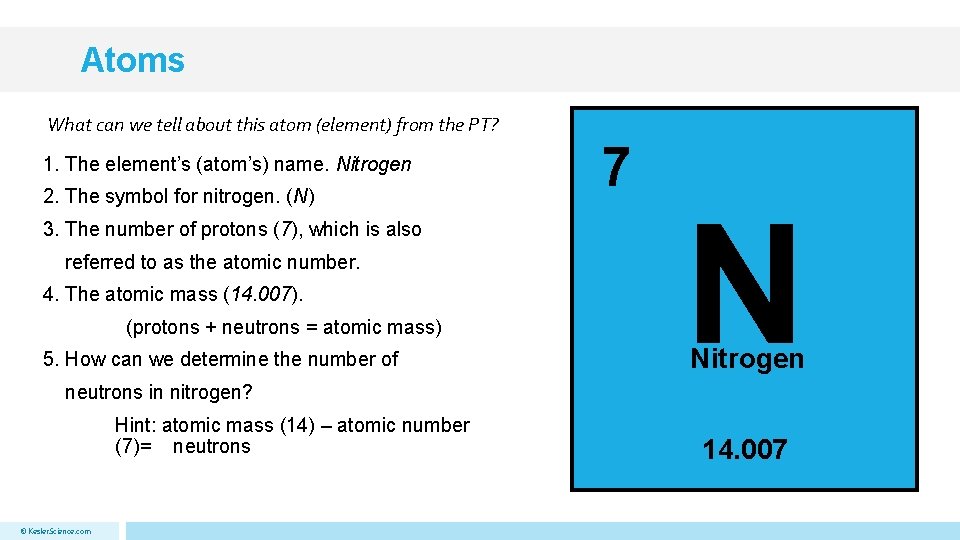
Atoms What can we tell about this atom (element) from the PT? 1. The element’s (atom’s) name. Nitrogen 2. The symbol for nitrogen. (N) 3. The number of protons (7), which is also referred to as the atomic number. 4. The atomic mass (14. 007). (protons + neutrons = atomic mass) 5. How can we determine the number of 7 N Nitrogen neutrons in nitrogen? Hint: atomic mass (14) – atomic number (7)= neutrons © Kesler. Science. com 14. 007
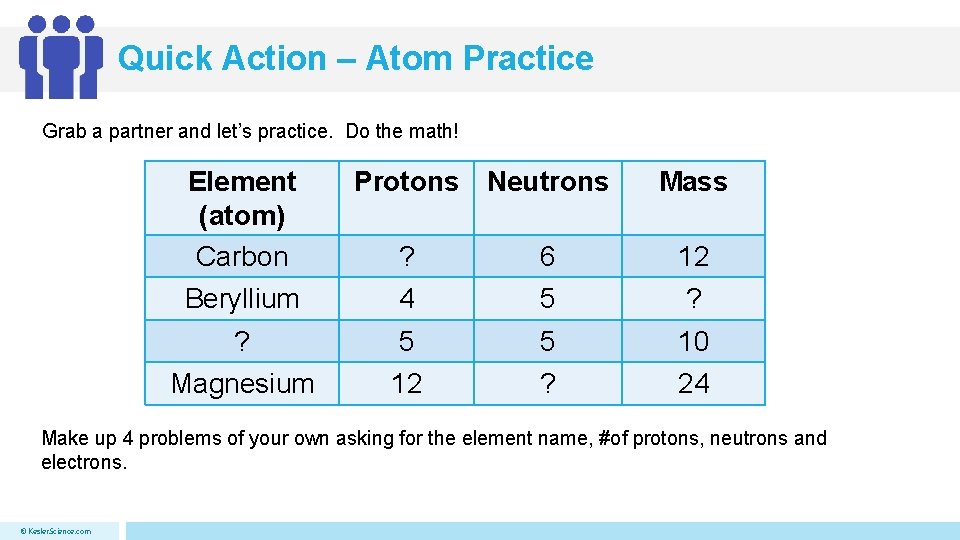
Quick Action – Atom Practice Grab a partner and let’s practice. Do the math! Element (atom) Carbon Protons Neutrons Mass ? 6 12 Beryllium 4 5 ? ? 5 5 10 Magnesium 12 ? 24 Make up 4 problems of your own asking for the element name, #of protons, neutrons and electrons. © Kesler. Science. com

Check for Understanding Can you… • Describe the structure of atoms, including the masses, electrical charges, and locations of protons, neutrons and electrons? • Identify an element by its number of protons? © Kesler. Science. com
- Slides: 17