Atoms Molecules and Stoichiometry Recap of Lecture 5


- Slides: 19

Atoms, Molecules, and Stoichiometry Recap of Lecture 5

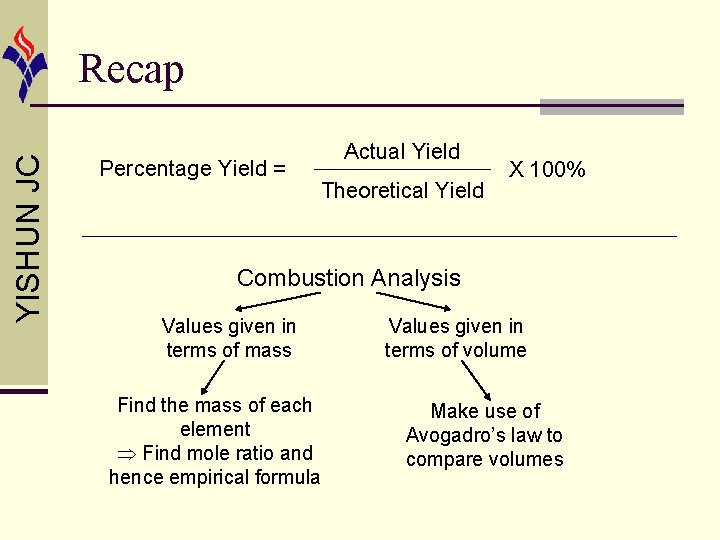
YISHUN JC Recap Percentage Yield = Actual Yield Theoretical Yield X 100% Combustion Analysis Values given in terms of mass Find the mass of each element Find mole ratio and hence empirical formula Values given in terms of volume Make use of Avogadro’s law to compare volumes

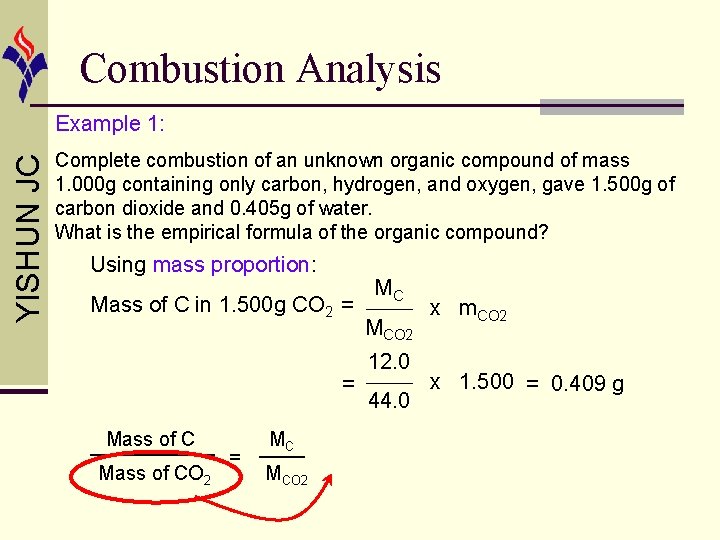
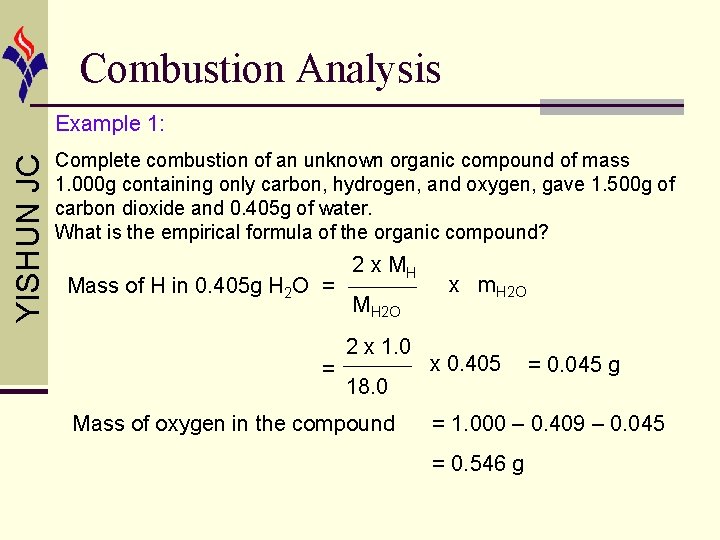
Combustion Analysis YISHUN JC Example 1: Complete combustion of an unknown organic compound of mass 1. 000 g containing only carbon, hydrogen, and oxygen, gave 1. 500 g of carbon dioxide and 0. 405 g of water. What is the empirical formula of the organic compound? Using mass proportion: Mass of C in 1. 500 g CO 2 = = Mass of CO 2 = MC MCO 2 12. 0 44. 0 x m. CO 2 x 1. 500 = 0. 409 g

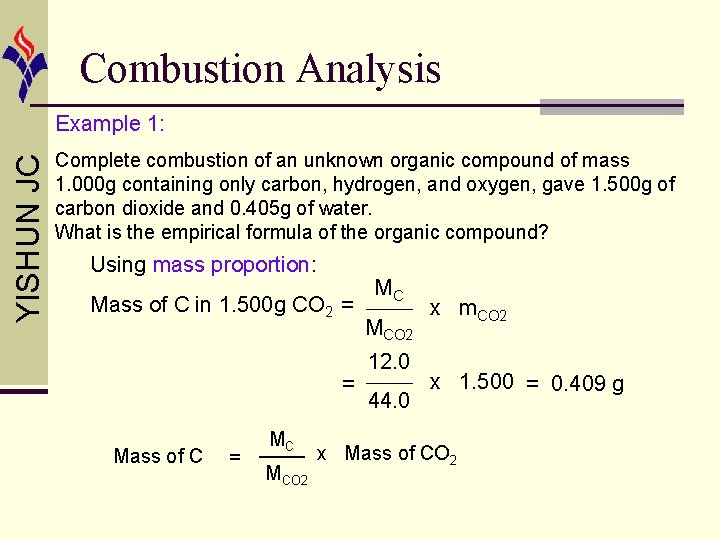
Combustion Analysis YISHUN JC Example 1: Complete combustion of an unknown organic compound of mass 1. 000 g containing only carbon, hydrogen, and oxygen, gave 1. 500 g of carbon dioxide and 0. 405 g of water. What is the empirical formula of the organic compound? Using mass proportion: Mass of C in 1. 500 g CO 2 = = Mass of C = MC MCO 2 12. 0 44. 0 x m. CO 2 x 1. 500 = 0. 409 g x Mass of CO 2

Combustion Analysis YISHUN JC Example 1: Complete combustion of an unknown organic compound of mass 1. 000 g containing only carbon, hydrogen, and oxygen, gave 1. 500 g of carbon dioxide and 0. 405 g of water. What is the empirical formula of the organic compound? Mass of H in 0. 405 g H 2 O = = 2 x MH MH 2 O 2 x 1. 0 18. 0 Mass of oxygen in the compound x m. H 2 O x 0. 405 = 0. 045 g = 1. 000 – 0. 409 – 0. 045 = 0. 546 g

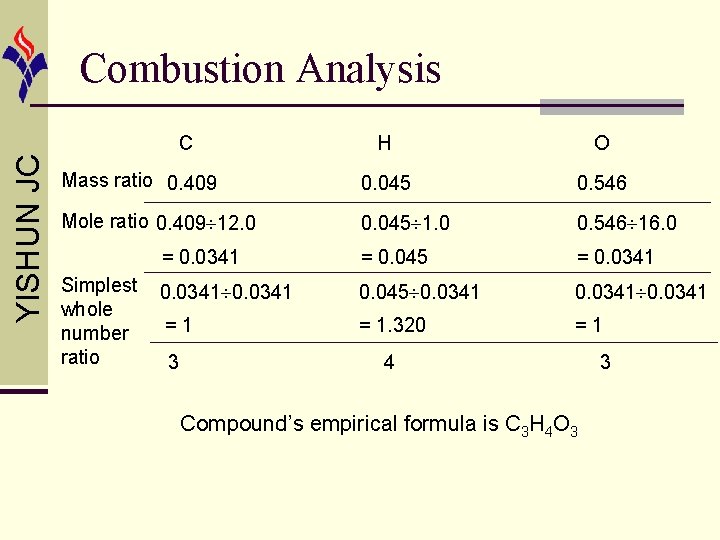
YISHUN JC Combustion Analysis C H O Mass ratio 0. 409 0. 045 0. 546 Mole ratio 0. 409 12. 0 0. 045 1. 0 0. 546 16. 0 = 0. 0341 = 0. 045 = 0. 0341 0. 045 0. 0341 =1 = 1. 320 =1 3 4 Simplest whole number ratio Compound’s empirical formula is C 3 H 4 O 3 3

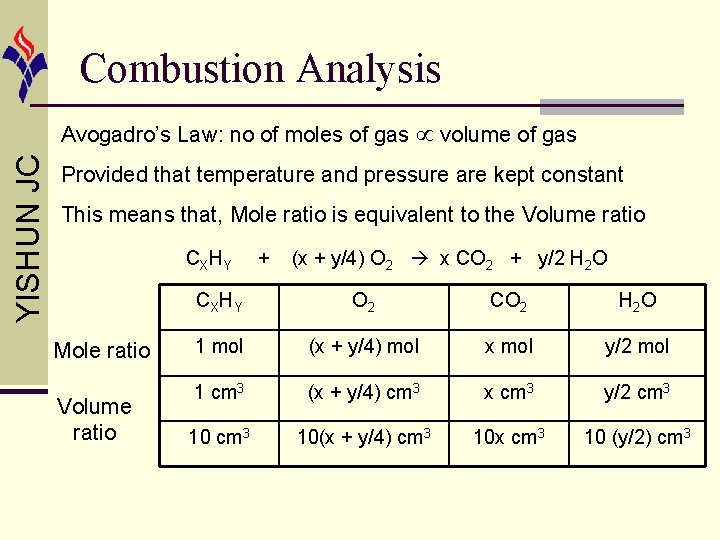
Combustion Analysis YISHUN JC Avogadro’s Law: no of moles of gas volume of gas Provided that temperature and pressure are kept constant This means that, Mole ratio is equivalent to the Volume ratio CXHY Mole ratio Volume ratio + (x + y/4) O 2 x CO 2 + y/2 H 2 O CXHY O 2 CO 2 H 2 O 1 mol (x + y/4) mol x mol y/2 mol 1 cm 3 (x + y/4) cm 3 x cm 3 y/2 cm 3 10(x + y/4) cm 3 10 x cm 3 10 (y/2) cm 3

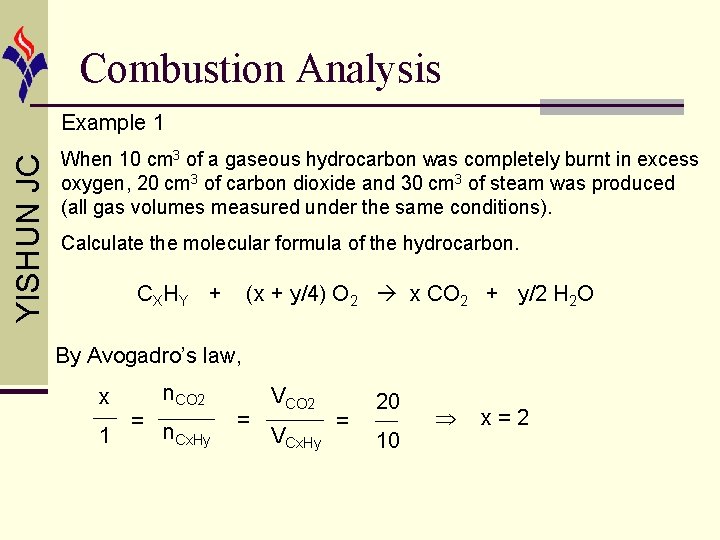
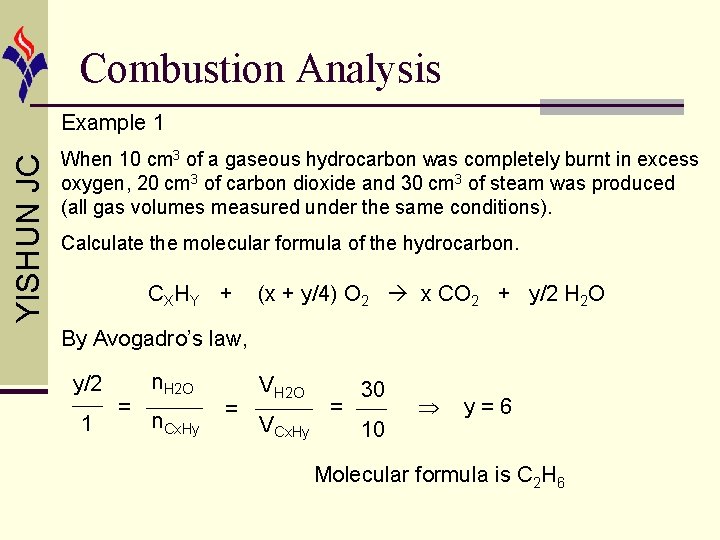
Combustion Analysis YISHUN JC Example 1 When 10 cm 3 of a gaseous hydrocarbon was completely burnt in excess oxygen, 20 cm 3 of carbon dioxide and 30 cm 3 of steam was produced (all gas volumes measured under the same conditions). Calculate the molecular formula of the hydrocarbon. CX HY + (x + y/4) O 2 x CO 2 + y/2 H 2 O By Avogadro’s law, x 1 = n. CO 2 n. Cx. Hy = VCO 2 VCx. Hy = 20 10 x=2

Combustion Analysis YISHUN JC Example 1 When 10 cm 3 of a gaseous hydrocarbon was completely burnt in excess oxygen, 20 cm 3 of carbon dioxide and 30 cm 3 of steam was produced (all gas volumes measured under the same conditions). Calculate the molecular formula of the hydrocarbon. CX HY + (x + y/4) O 2 x CO 2 + y/2 H 2 O By Avogadro’s law, y/2 1 = n. H 2 O n. Cx. Hy = VH 2 O VCx. Hy = 30 10 y=6 Molecular formula is C 2 H 6

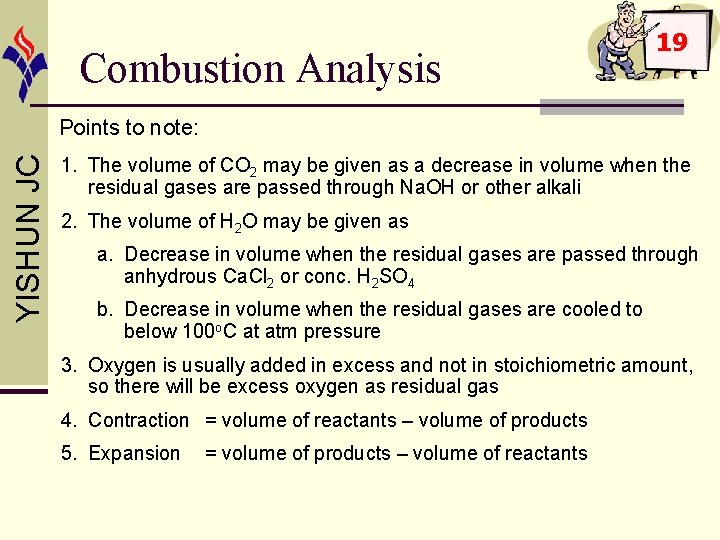
Combustion Analysis 19 YISHUN JC Points to note: 1. The volume of CO 2 may be given as a decrease in volume when the residual gases are passed through Na. OH or other alkali 2. The volume of H 2 O may be given as a. Decrease in volume when the residual gases are passed through anhydrous Ca. Cl 2 or conc. H 2 SO 4 b. Decrease in volume when the residual gases are cooled to below 100 o. C at atm pressure 3. Oxygen is usually added in excess and not in stoichiometric amount, so there will be excess oxygen as residual gas 4. Contraction = volume of reactants – volume of products 5. Expansion = volume of products – volume of reactants

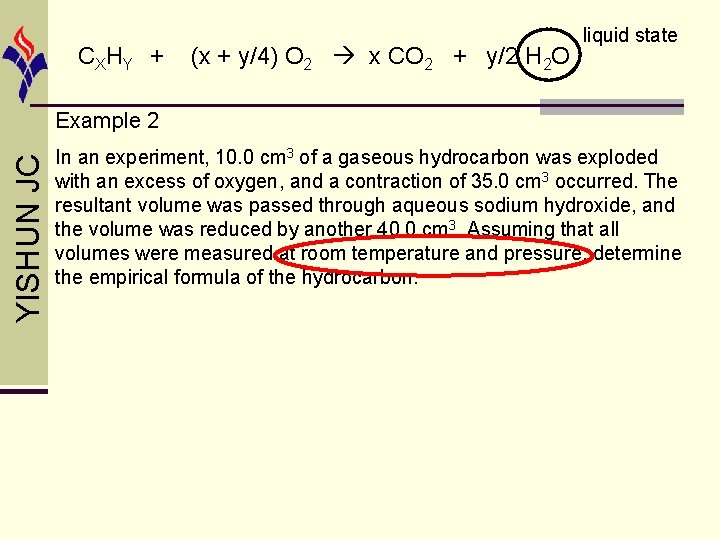
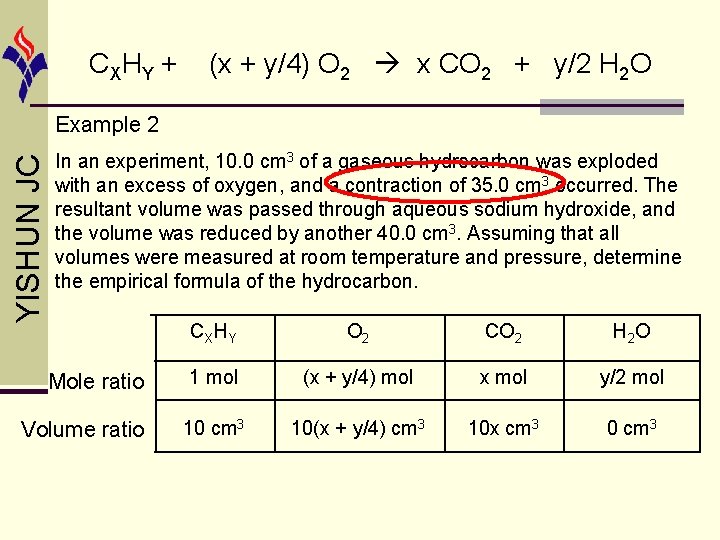
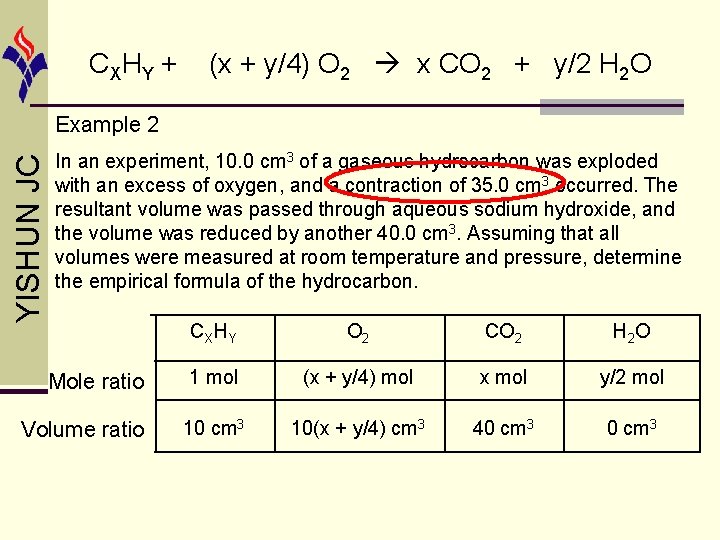
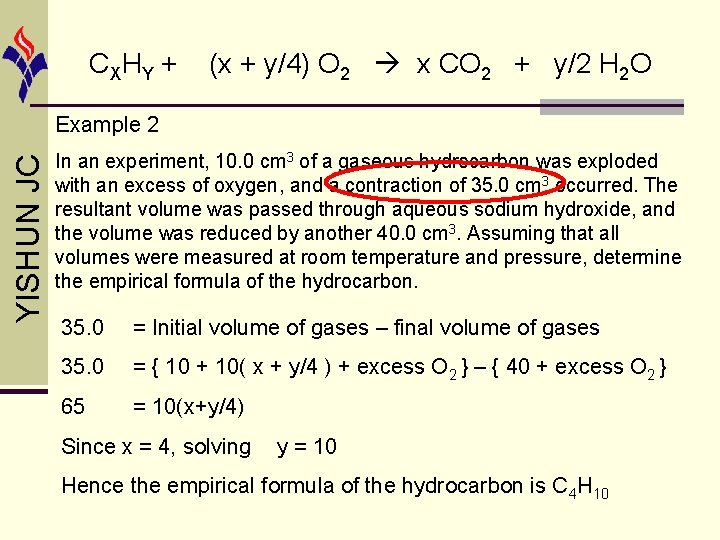
CX HY + (x + y/4) O 2 x CO 2 + y/2 H 2 O liquid state YISHUN JC Example 2 In an experiment, 10. 0 cm 3 of a gaseous hydrocarbon was exploded with an excess of oxygen, and a contraction of 35. 0 cm 3 occurred. The resultant volume was passed through aqueous sodium hydroxide, and the volume was reduced by another 40. 0 cm 3. Assuming that all volumes were measured at room temperature and pressure, determine the empirical formula of the hydrocarbon.

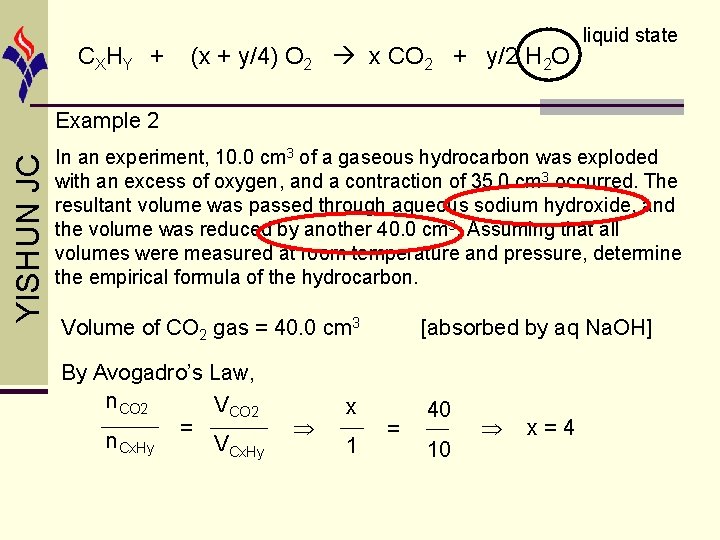
CX HY + (x + y/4) O 2 x CO 2 + y/2 H 2 O liquid state YISHUN JC Example 2 In an experiment, 10. 0 cm 3 of a gaseous hydrocarbon was exploded with an excess of oxygen, and a contraction of 35. 0 cm 3 occurred. The resultant volume was passed through aqueous sodium hydroxide, and the volume was reduced by another 40. 0 cm 3. Assuming that all volumes were measured at room temperature and pressure, determine the empirical formula of the hydrocarbon. Volume of CO 2 gas = 40. 0 cm 3 [absorbed by aq Na. OH] By Avogadro’s Law, n. CO 2 VCO 2 = n. Cx. Hy VCx. Hy 40 x 1 = 10 x=4

CX HY + (x + y/4) O 2 x CO 2 + y/2 H 2 O YISHUN JC Example 2 In an experiment, 10. 0 cm 3 of a gaseous hydrocarbon was exploded with an excess of oxygen, and a contraction of 35. 0 cm 3 occurred. The resultant volume was passed through aqueous sodium hydroxide, and the volume was reduced by another 40. 0 cm 3. Assuming that all volumes were measured at room temperature and pressure, determine the empirical formula of the hydrocarbon. Mole ratio Volume ratio CXHY O 2 CO 2 H 2 O 1 mol (x + y/4) mol x mol y/2 mol 10 cm 3 10(x + y/4) cm 3 10 x cm 3 0 cm 3

CX HY + (x + y/4) O 2 x CO 2 + y/2 H 2 O YISHUN JC Example 2 In an experiment, 10. 0 cm 3 of a gaseous hydrocarbon was exploded with an excess of oxygen, and a contraction of 35. 0 cm 3 occurred. The resultant volume was passed through aqueous sodium hydroxide, and the volume was reduced by another 40. 0 cm 3. Assuming that all volumes were measured at room temperature and pressure, determine the empirical formula of the hydrocarbon. Mole ratio Volume ratio CXHY O 2 CO 2 H 2 O 1 mol (x + y/4) mol x mol y/2 mol 10 cm 3 10(x + y/4) cm 3 40 cm 3

CX HY + (x + y/4) O 2 x CO 2 + y/2 H 2 O YISHUN JC Example 2 In an experiment, 10. 0 cm 3 of a gaseous hydrocarbon was exploded with an excess of oxygen, and a contraction of 35. 0 cm 3 occurred. The resultant volume was passed through aqueous sodium hydroxide, and the volume was reduced by another 40. 0 cm 3. Assuming that all volumes were measured at room temperature and pressure, determine the empirical formula of the hydrocarbon. 35. 0 = Initial volume of gases – final volume of gases 35. 0 = { 10 + 10( x + y/4 ) + excess O 2 } – { 40 + excess O 2 } 65 = 10(x+y/4) Since x = 4, solving y = 10 Hence the empirical formula of the hydrocarbon is C 4 H 10

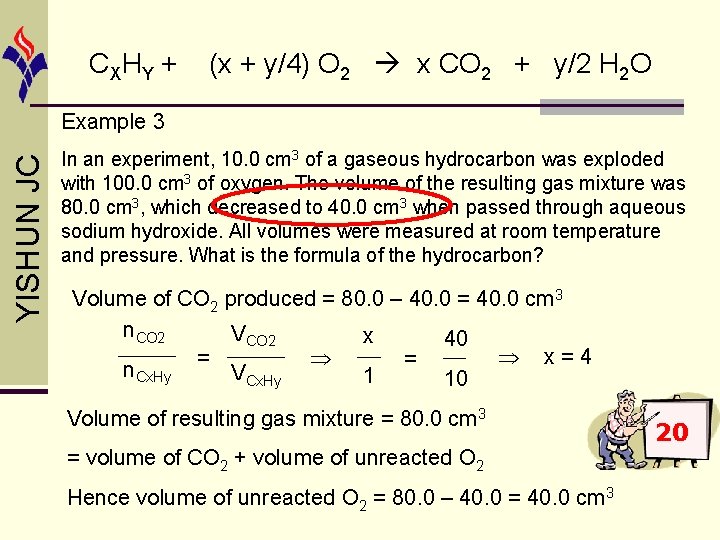
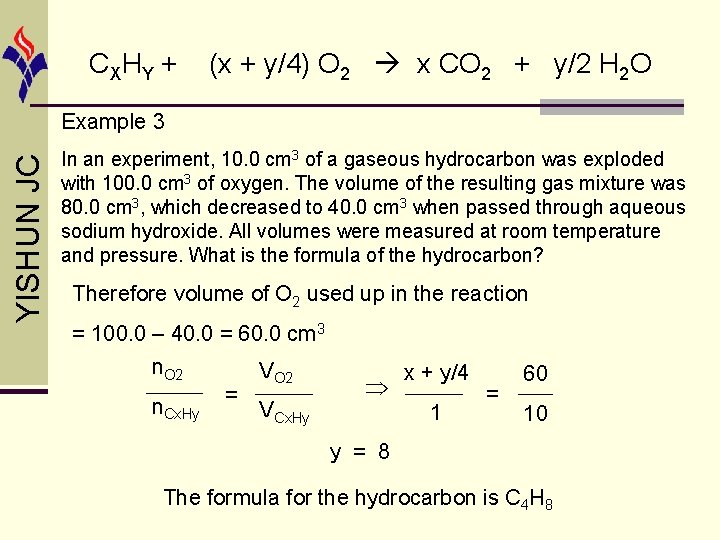
CX HY + (x + y/4) O 2 x CO 2 + y/2 H 2 O YISHUN JC Example 3 In an experiment, 10. 0 cm 3 of a gaseous hydrocarbon was exploded with 100. 0 cm 3 of oxygen. The volume of the resulting gas mixture was 80. 0 cm 3, which decreased to 40. 0 cm 3 when passed through aqueous sodium hydroxide. All volumes were measured at room temperature and pressure. What is the formula of the hydrocarbon? Volume of CO 2 produced = 80. 0 – 40. 0 = 40. 0 cm 3 n. CO 2 VCO 2 x 40 x=4 = = n. Cx. Hy VCx. Hy 1 10 Volume of resulting gas mixture = 80. 0 cm 3 = volume of CO 2 + volume of unreacted O 2 Hence volume of unreacted O 2 = 80. 0 – 40. 0 = 40. 0 cm 3 20

CX HY + (x + y/4) O 2 x CO 2 + y/2 H 2 O YISHUN JC Example 3 In an experiment, 10. 0 cm 3 of a gaseous hydrocarbon was exploded with 100. 0 cm 3 of oxygen. The volume of the resulting gas mixture was 80. 0 cm 3, which decreased to 40. 0 cm 3 when passed through aqueous sodium hydroxide. All volumes were measured at room temperature and pressure. What is the formula of the hydrocarbon? Therefore volume of O 2 used up in the reaction = 100. 0 – 40. 0 = 60. 0 cm 3 n. O 2 n. Cx. Hy = VO 2 VCx. Hy x + y/4 1 = 60 10 y = 8 The formula for the hydrocarbon is C 4 H 8

What have I learnt? YISHUN JC n Determine the formula of a hydrocarbon given the combustion analysis data n In terms of mass n In terms of volume

End of Lecture 6 (End of part I) Have a prosperous and happy lunar new year (and do take care of your health!)