Atoms Molecules and Matter Copy the Concept Map


![Diatomic Molecules NOFCl. Br. I [H] Nitrogen Bromine Oxygen Iodine Fluorine Hydrogen Chlorine Diatomic Molecules NOFCl. Br. I [H] Nitrogen Bromine Oxygen Iodine Fluorine Hydrogen Chlorine](https://slidetodoc.com/presentation_image_h2/e485e959e4d04e325751667b9668ce4f/image-10.jpg)




- Slides: 20

Atoms, Molecules and Matter Copy the Concept Map to the Right and Fill it in with the words below: ATOMS COMPOUNDS MATTER Is composed of… Bond together to form…

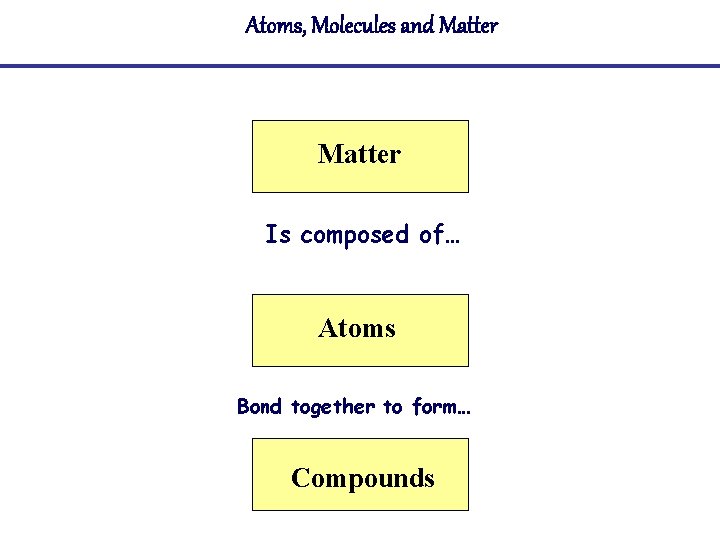
Atoms, Molecules and Matter Is composed of… Atoms Bond together to form… Compounds

Matter • Matter is the “stuff” of which the universe is composed. • Two characteristics: has mass and occupies space.

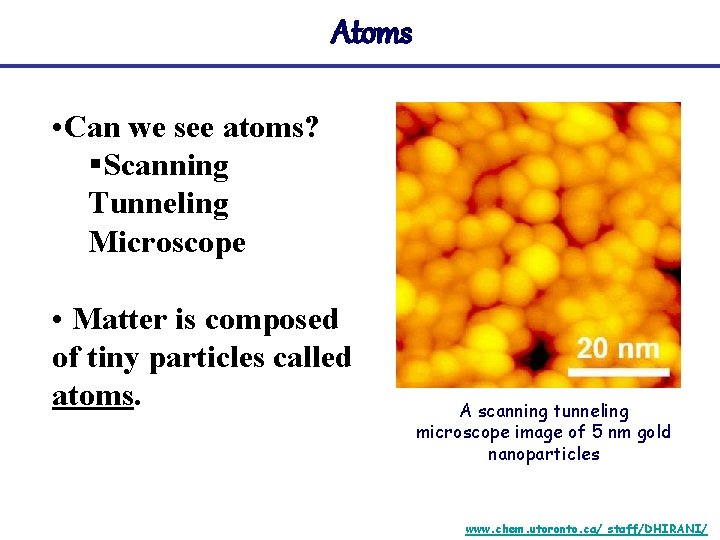
Atoms • Can we see atoms? §Scanning Tunneling Microscope • Matter is composed of tiny particles called atoms. A scanning tunneling microscope image of 5 nm gold nanoparticles www. chem. utoronto. ca/ staff/DHIRANI/

Compound vs Molecules • A molecule is formed when two or more atoms join together chemically. • A compound is a molecule that contains at least two different elements. • All compounds are molecules but not all molecules are compounds.

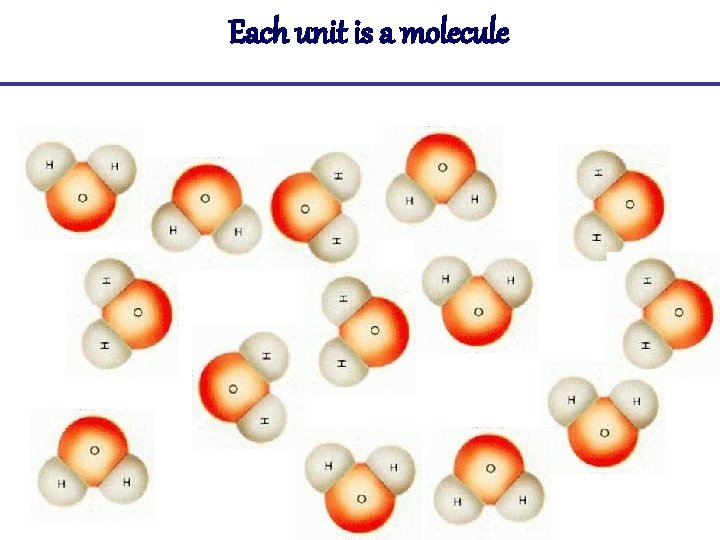
Each unit is a molecule

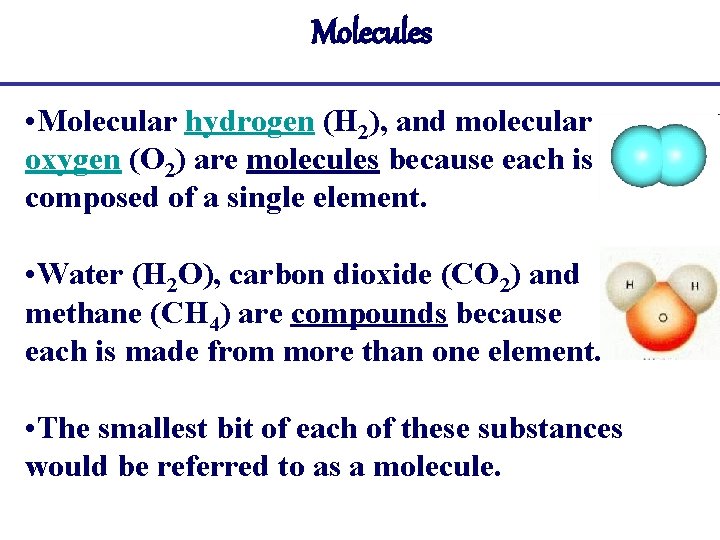
Molecules • Molecular hydrogen (H 2), and molecular oxygen (O 2) are molecules because each is composed of a single element. • Water (H 2 O), carbon dioxide (CO 2) and methane (CH 4) are compounds because each is made from more than one element. • The smallest bit of each of these substances would be referred to as a molecule.

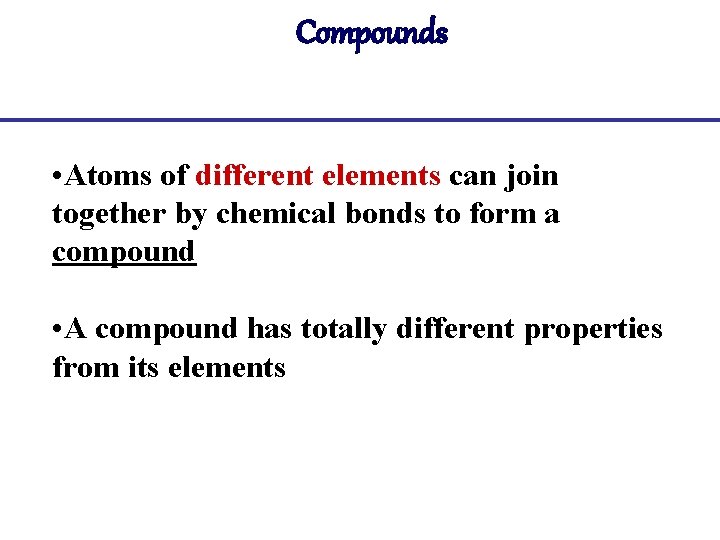
Compounds • Atoms of different elements can join together by chemical bonds to form a compound • A compound has totally different properties from its elements

Sodium Chloride • Sodium: Silvery White Metal http: //en. wikipedia. org/wiki/Sodium Chlorine: Pale Green Gas http: //en. wikipedia. org/wiki/Chlorine
![Diatomic Molecules NOFCl Br I H Nitrogen Bromine Oxygen Iodine Fluorine Hydrogen Chlorine Diatomic Molecules NOFCl. Br. I [H] Nitrogen Bromine Oxygen Iodine Fluorine Hydrogen Chlorine](https://slidetodoc.com/presentation_image_h2/e485e959e4d04e325751667b9668ce4f/image-10.jpg)
Diatomic Molecules NOFCl. Br. I [H] Nitrogen Bromine Oxygen Iodine Fluorine Hydrogen Chlorine

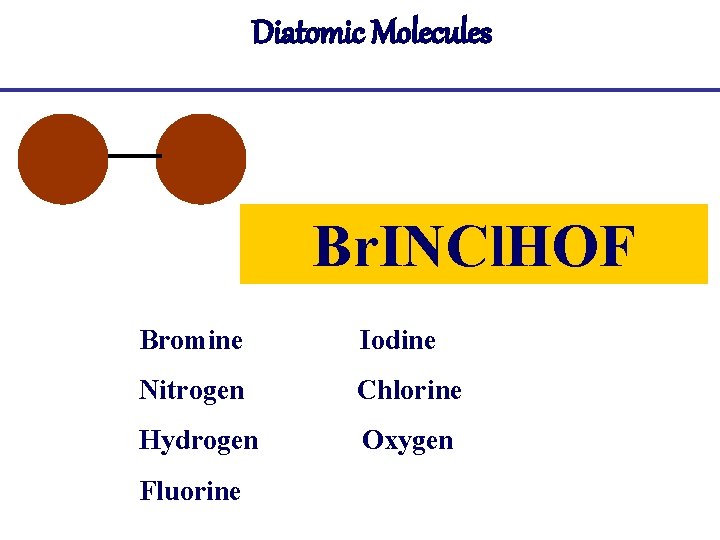
Diatomic Molecules Br. INCl. HOF Bromine Iodine Nitrogen Chlorine Hydrogen Oxygen Fluorine

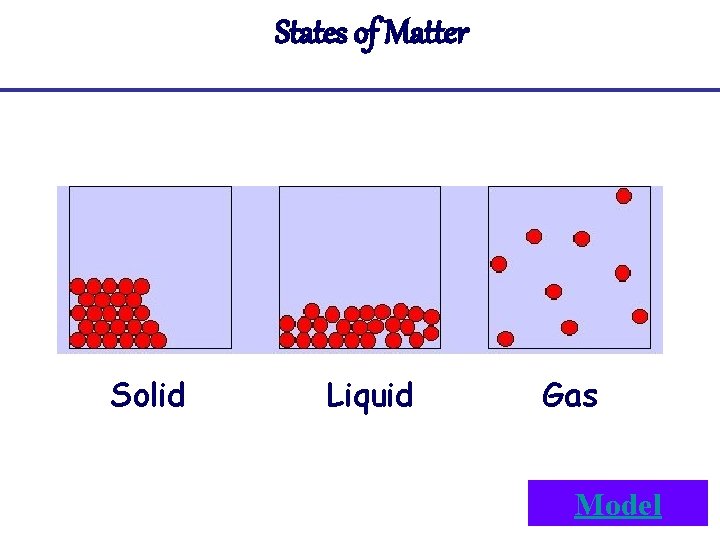
States of Matter Solid Liquid Gas Model

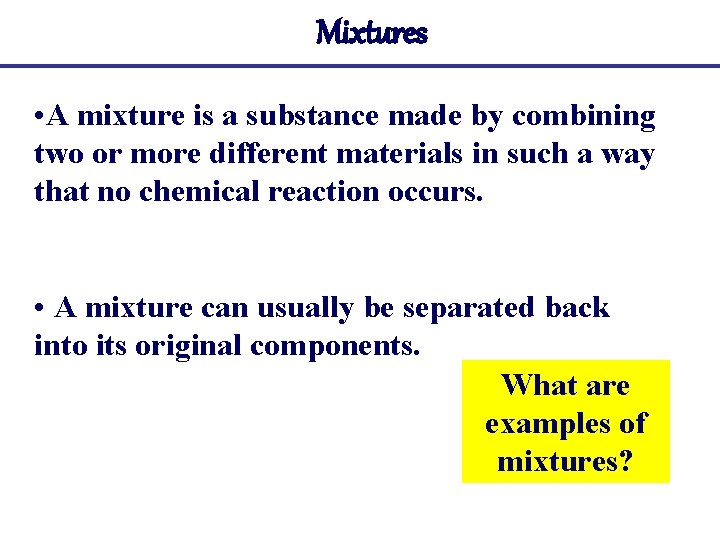
Mixtures • A mixture is a substance made by combining two or more different materials in such a way that no chemical reaction occurs. • A mixture can usually be separated back into its original components. What are examples of mixtures?

Prefixes • Homo: • Greek = Same • Latin = Human • Hetero: • Greek = Mixed What words using these prefixes come to mind?

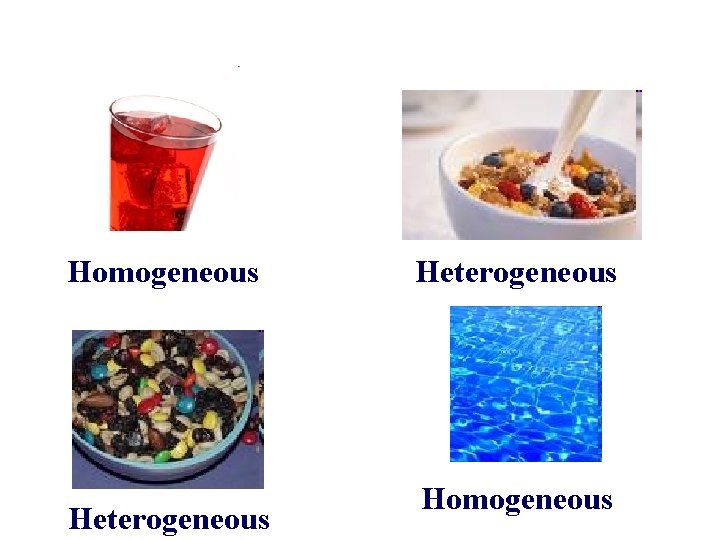
Mixtures • Homogenous Mixture: the particles are mixed so well that the separate parts cannot be seen (Salt Water) • Heterogenous Mixture: the different parts can be easily seen (Salt and Pepper)

Sand

Sand close up collections. ic. gc. ca/. . . / magnified-sand_sa. htm

Homogeneous Heterogeneous Homogeneous

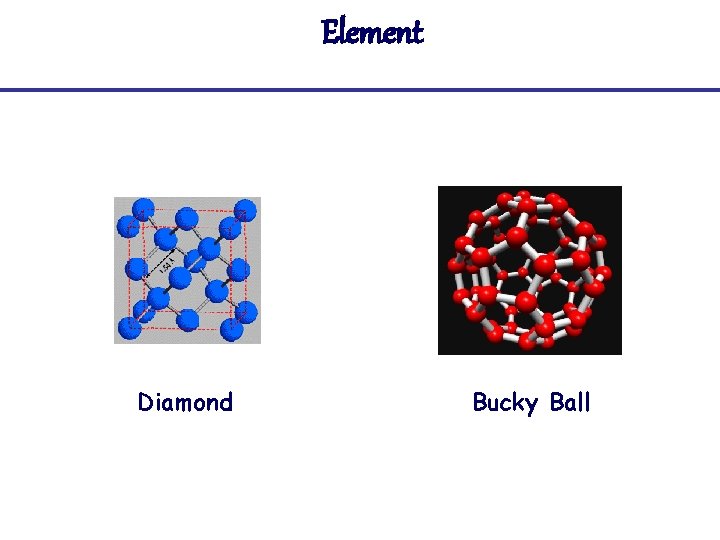
Element Diamond Bucky Ball

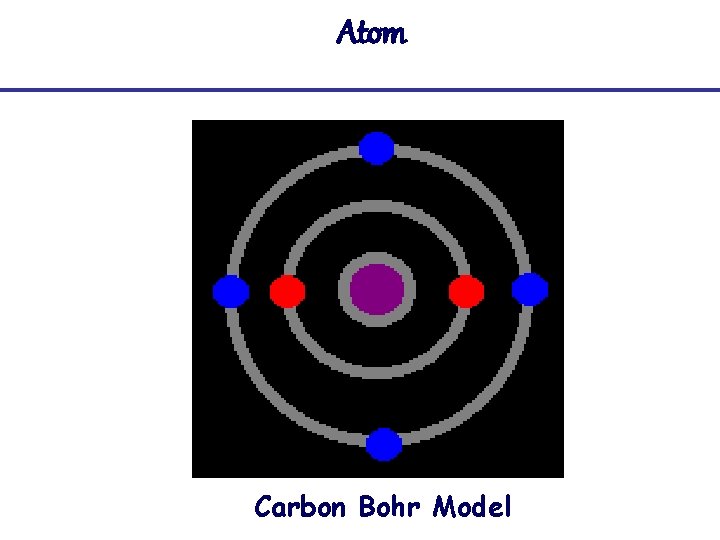
Atom Carbon Bohr Model