Atoms Ions and Isotopes Subatomic Particles Particle Symbol

Atoms, Ions and Isotopes
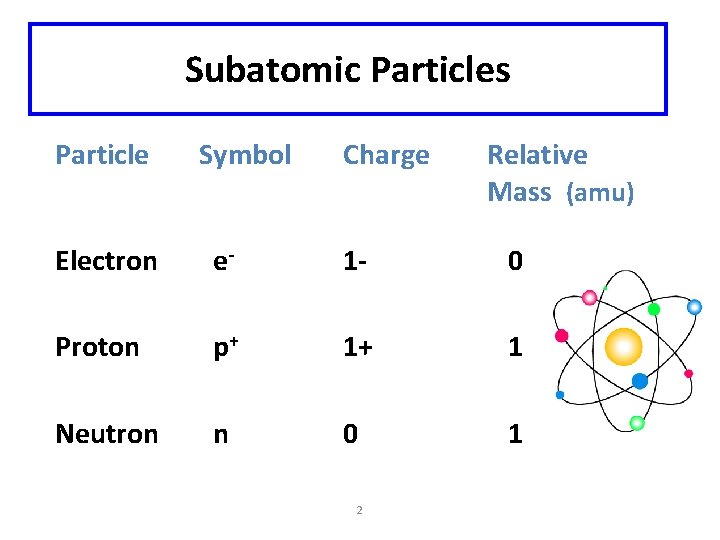
Subatomic Particles Particle Symbol Charge Relative Mass (amu) Electron e- 1 - 0 Proton p+ 1+ 1 Neutron n 0 1 2
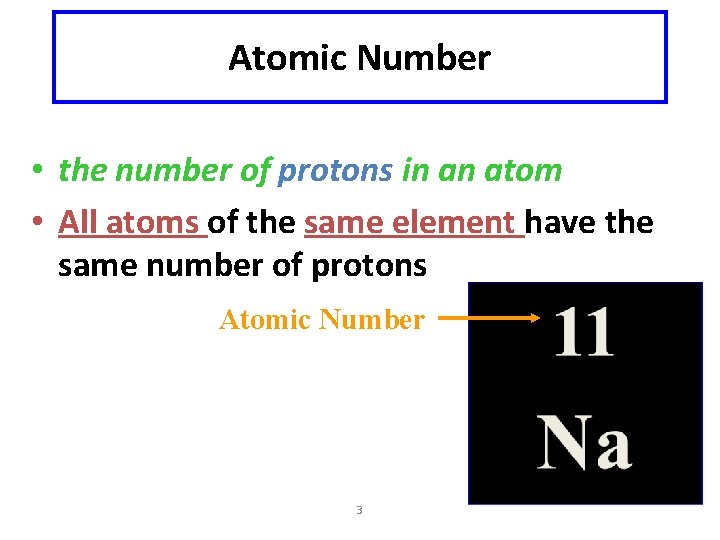
Atomic Number • the number of protons in an atom • All atoms of the same element have the same number of protons Atomic Number 3
Number of Electrons • If an atom is neutral, the net charge is zero – Number of protons = Number of electrons 4
Mass Number = protons + neutrons • number of neutrons = mass number – atomic number *Mass number is NOT on the periodic table 5
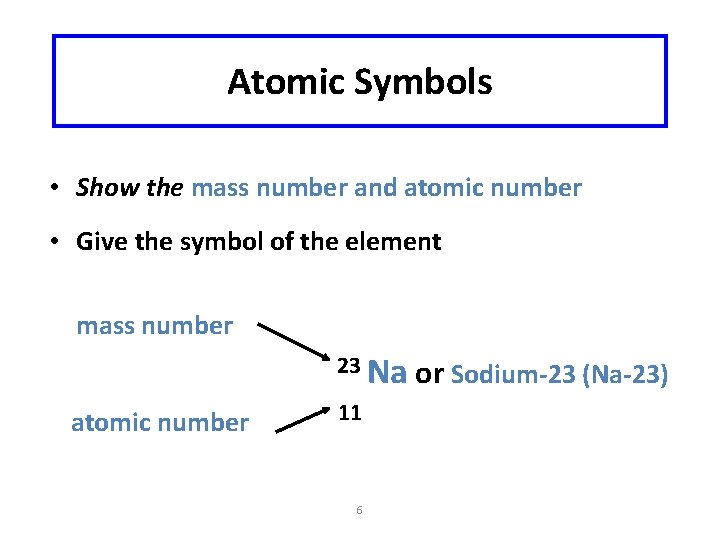
Atomic Symbols • Show the mass number and atomic number • Give the symbol of the element mass number 23 Na atomic number 11 6 or Sodium-23 (Na-23)
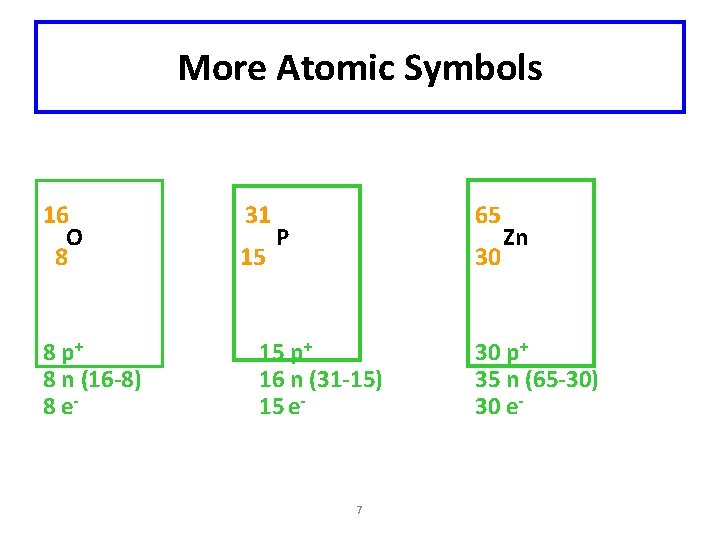
More Atomic Symbols 16 O 8 8 p+ 8 n (16 -8) 8 e- 31 15 65 P 30 15 p+ 16 n (31 -15) 15 e- 7 Zn 30 p+ 35 n (65 -30) 30 e-
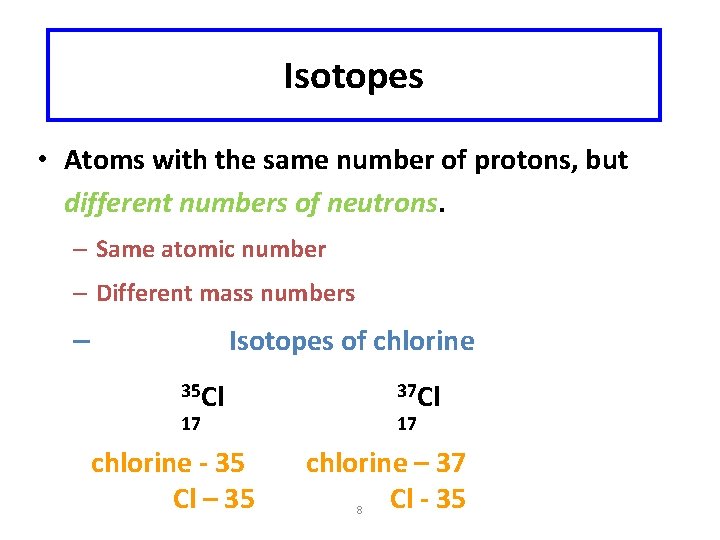
Isotopes • Atoms with the same number of protons, but different numbers of neutrons. – Same atomic number – Different mass numbers Isotopes of chlorine – 35 Cl 37 Cl 17 chlorine - 35 Cl – 35 17 chlorine – 37 Cl - 35 8

Ions • An atom that has gained or lost electrons – An atom that loses electrons has a positive charge (+) • Positive ions are called cations • ex. Na+, Mg+2 – An atoms that gains electrons has a negative charge • Negative ions are called anions • Ex. Cl-, O-2 • How many protons, neutrons, and electrons are there in the Calcium ion (Ca+2)? • How many protons, neutrons, and electrons are there in the Fluoride ion (F -)?
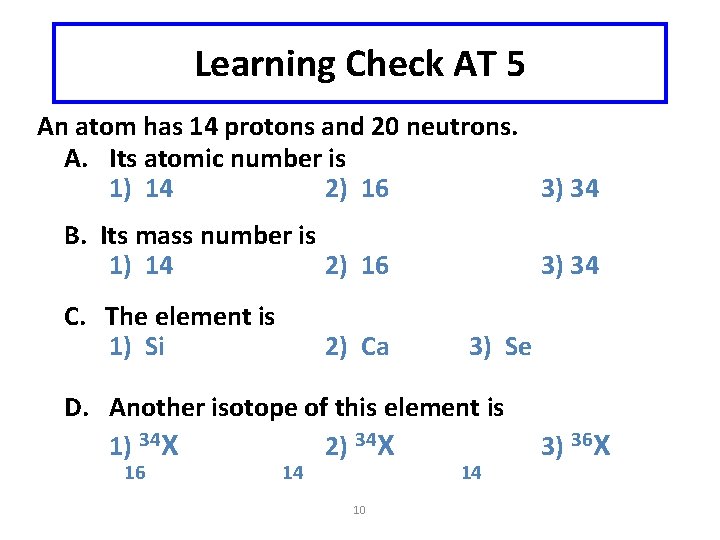
Learning Check AT 5 An atom has 14 protons and 20 neutrons. A. Its atomic number is 1) 14 2) 16 3) 34 B. Its mass number is 1) 14 2) 16 C. The element is 1) Si 2) Ca 3) 34 3) Se D. Another isotope of this element is 1) 34 X 2) 34 X 16 14 14 10 3) 36 X
Masses of Atoms • A scale designed for atoms gives their small atomic masses in atomic mass units (amu) • An atom of 12 C was assigned an exact mass of 12. 00 amu • Relative masses of all other atoms was determined by comparing each to the mass of 12 C 11
Calculating Average Atomic Mass • You need to know… – Percent(%) abundance of isotopes – Mass of each isotope • Weighted average = (mass of isotope 1)(% abundance/100) +(mass of isotope 2)(% abundance/100) + … 12
Atomic Mass of Magnesium Isotopes 24 Mg = Mass of Isotope Abundance 24. 0 amu 78. 70% 25 Mg = 25. 0 amu 10. 13% = 26. 0 amu 11. 17% 26 Mg (. 7870 x 24. 0) + (. 1013 x 25. 0) + (. 1117 x 26. 0) Atomic mass (average mass) Mg = 24. 3 amu Mg 24. 3 13
Learning Check AT 7 Gallium is a metallic element found in small lasers used in compact disc players. In a sample of gallium, there is 60. 2% of gallium-69 (68. 9 amu) atoms and 39. 8% of gallium-71 (70. 9 amu) atoms. What is the average atomic mass of gallium? 14
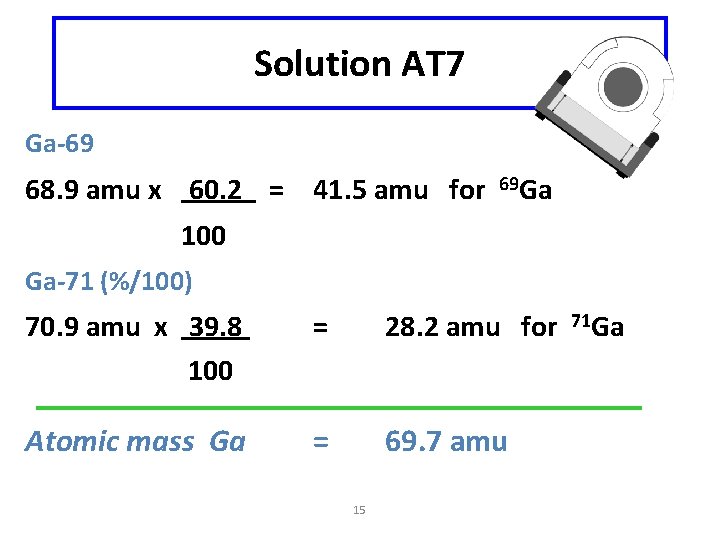
Solution AT 7 Ga-69 68. 9 amu x 60. 2 = 41. 5 amu for 69 Ga 100 Ga-71 (%/100) 70. 9 amu x 39. 8 100 = 28. 2 amu for Atomic mass Ga = 69. 7 amu 15 71 Ga
- Slides: 15