Atoms Elements Molecules Compounds ATOM An atom is
































- Slides: 53

Atoms Elements Molecules Compounds

ATOM An atom is the smallest particle into which an element can be divided and still be the same substance.

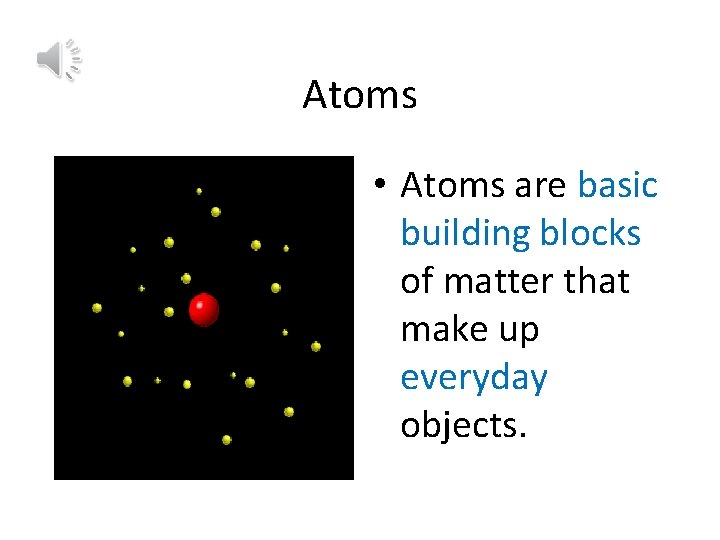
Atoms • Atoms are basic building blocks of matter that make up everyday objects.

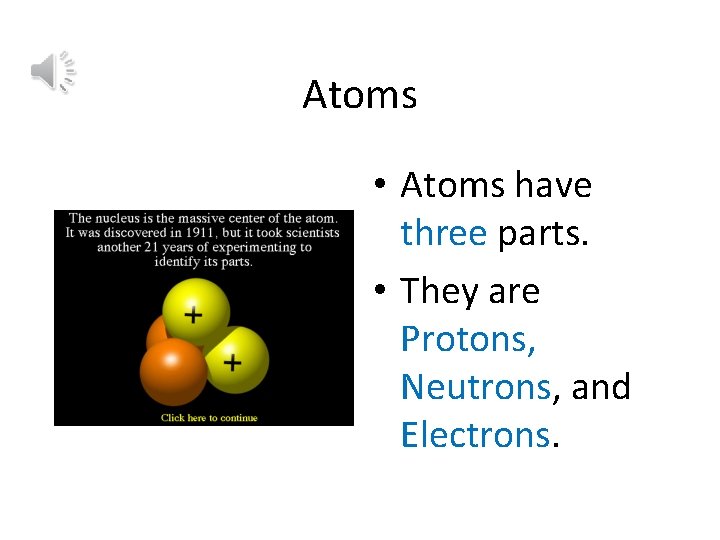
Atoms • Atoms have three parts. • They are Protons, Neutrons, and Electrons.

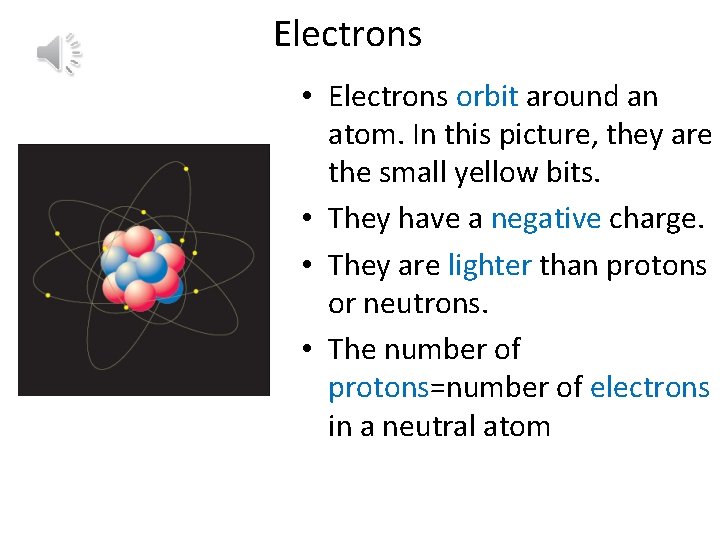
Electrons • Electrons orbit around an atom. In this picture, they are the small yellow bits. • They have a negative charge. • They are lighter than protons or neutrons. • The number of protons=number of electrons in a neutral atom

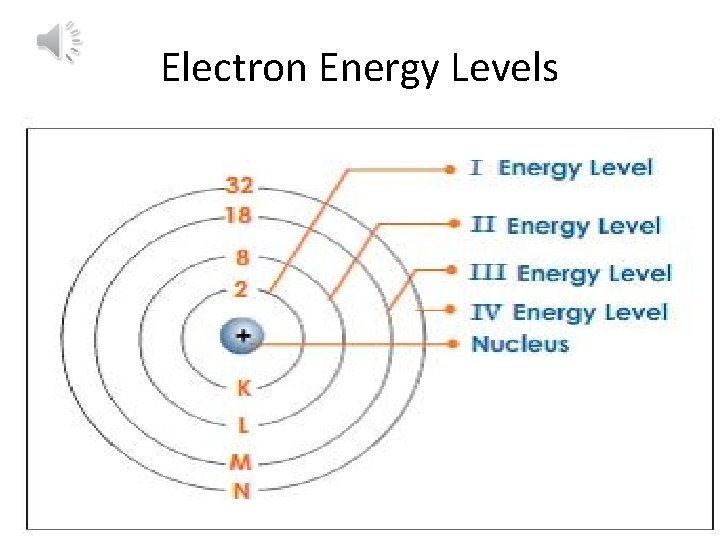
Electron Energy Levels

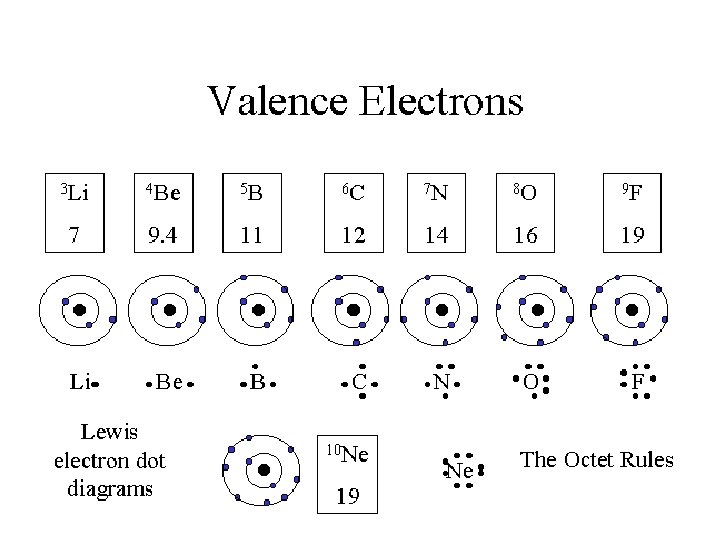
Valance Electrons • Are the electrons in the last shell or energy level of an atom. • They do show a repeating or periodic pattern. • The valence electrons increase in number as you go across a period. • Elements in the same column on the periodic table have the same number of valance electrons.




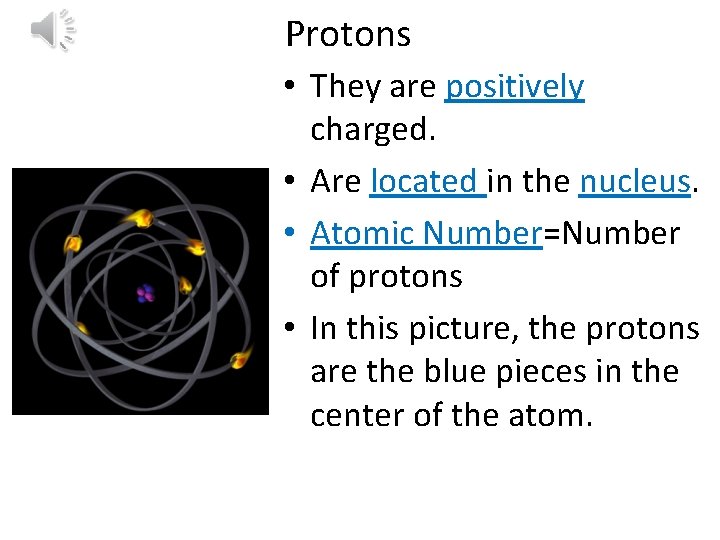
Protons • They are positively charged. • Are located in the nucleus. • Atomic Number=Number of protons • In this picture, the protons are the blue pieces in the center of the atom.

Neutrons • Neutrons are neither positive nor negative. • Neutrons are in the nucleus of an atom. • The atomic mass – number of protons = number of neutrons • In this picture, neutrons are the purple pieces in the center of the atom.

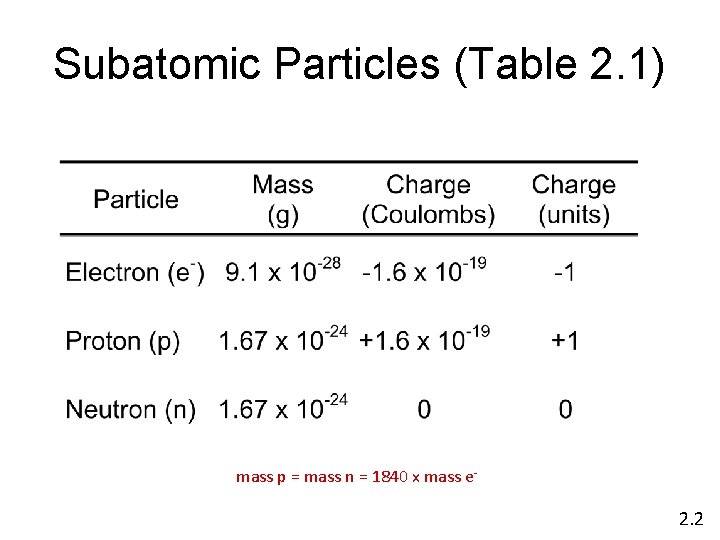
Subatomic Particles (Table 2. 1) mass p = mass n = 1840 x mass e- 2. 2

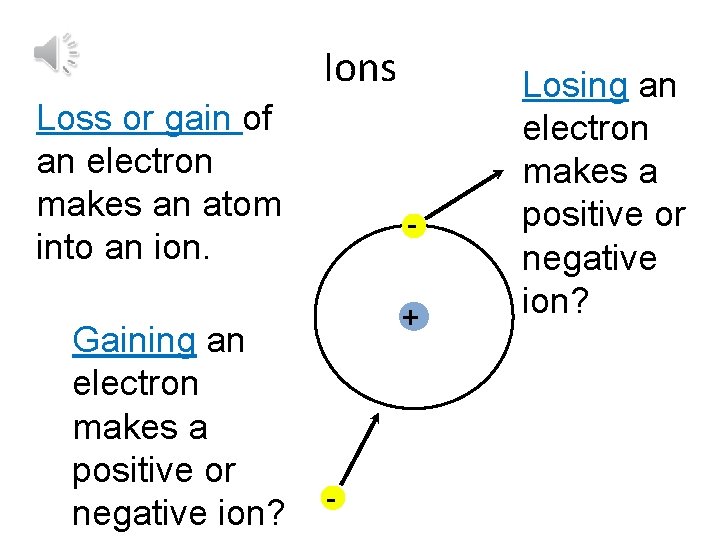
Ions Loss or gain of an electron makes an atom into an ion. Gaining an electron makes a positive or negative ion? + - Losing an electron makes a positive or negative ion?

Isotopes Atoms that gain or lose a neutron become isotopes. Radioactive isotopes are used in medicine for imaging (such as PET scanners).

https: //www. youtube. com/watch? v=cn. XV 7 Ph 3 WPk https: //www. youtube. com/watch? v=d 3 YR 8 e_i. Hlk https: //www. youtube. com/watch? v=D-i. PPw. DAk 1 Q https: //www. youtube. com/watch? v=Le 9 a 8 C 0 Pk 2 c

History of the Atomic Theory

Dalton John Dalton developed his atomic theory from observations gathered from many experiments. http: //www. britannica. com/eb/art/print? id=15469&article. Type. Id=0

Dalton’s ATOMIC THEORY 1. All substances are made of atoms. Atoms are small particles that cannot be created, divided, or destroyed.

Dalton’s ATOMIC THEORY 2. Atoms of the same element are exactly alike, and atoms of different elements are different.

Dalton’s ATOMIC THEORY 3. Atoms join with other atoms to make new substances.

J. J. Thomson o In 1897, J. J. Thomson made a discovery that identified an error in Dalton’s theory. o There are small particles inside the atom.

Thomson’s Results o Thomson discovered atoms can be divided into even smaller parts. o What he discovered was the “electron. ” Thomson called it a “corpuscle” back then.

Revision of the Atomic Theory Because Thomson knew atoms have no overall charge, he realized that positive charges must be present to balance the negative charges of the electron.

Rutherford (1909) Ernest Rutherford, a former student of Thomson, designed an experiment to investigate the structure of the atom. http: //www. pbs. org/wgbh/aso/databank/entries/bpruth. html

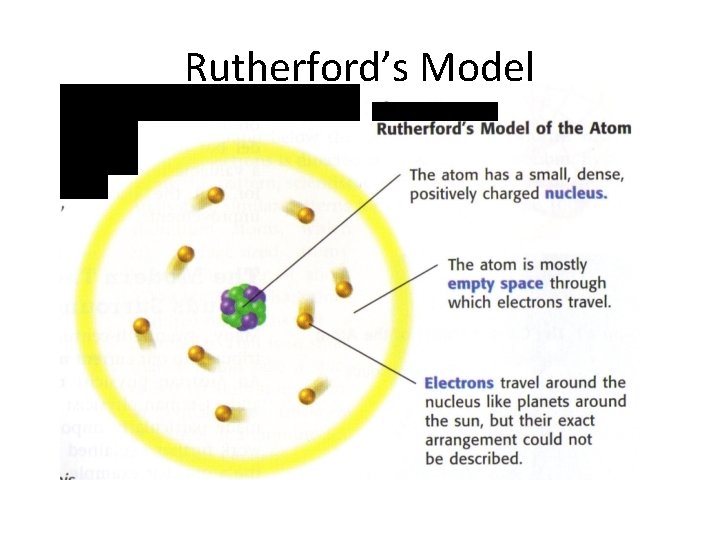
Revision of the Atomic Model Rutherford proposed the center of the atom is a tiny, extremely dense, positively charged region called the “NUCLEUS. ”

Rutherford’s Model

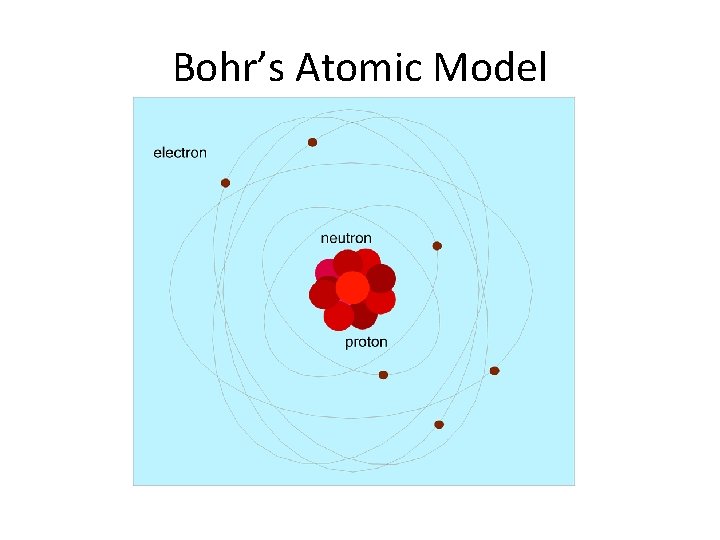
Bohr (1913) Neils Bohr was a Danish scientist who worked with Rutherford. He suggested electrons travel around the nucleus in definite paths. http: //www. pbs. org/wgbh/aso/databank/entries/bpbohr. html

These paths are located in levels at certain distances away from the nucleus.


Bohr’s Atomic Model

Chadwick (1932) James Chadwick, an English scientist, discovered the “NEUTRON. ” He won the Nobel Prize for Physics in 1935, and his research prepared the way for the development of the atomic bomb. http: //nobelprize. org/nobel_prizes/physics/laureates/1935/chadwick-bio. html

Still more changes… Many 20 th century scientists have contributed to our current understanding of the atom. http: //particleadventure. org/frameless/modern_atom. html

Electrons do not travel in definite paths around the nucleus like Bohr thought This discovery was made by scientists Erwin Schrodinger and Werner Heisenberg.

In the modern atomic theory… The exact path of a moving electron cannot be predicted, but there are regions inside the atom where electrons are likely to be found. These regions are called electron clouds. https: //www. youtube. com/watch? v=07 y. Di. ELe 83 Y

Elements

What is a Pure Substance? • A pure substance is a classification of matter that includes both elements and compounds • Pure substances cannot be separated by physical means such as distillation, filtration, or chromatography

Elements • An element is a substance made up of only one kind of atom. • Elements make up all substances on the earth.

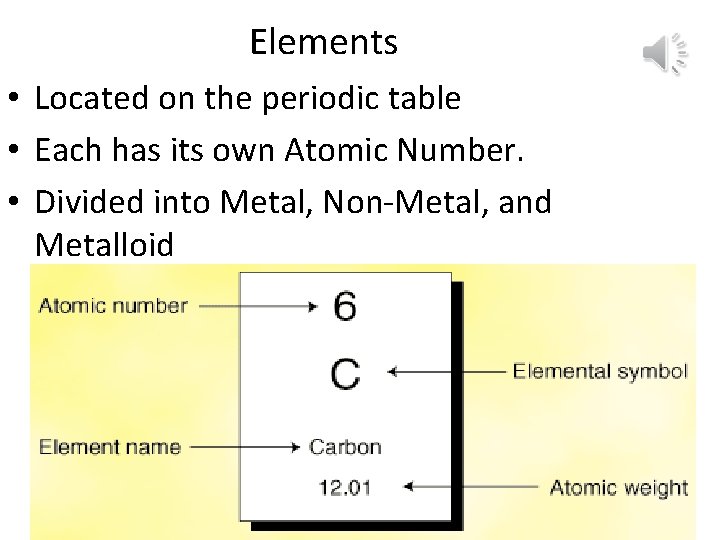
Elements • Located on the periodic table • Each has its own Atomic Number. • Divided into Metal, Non-Metal, and Metalloid


https: //www. youtube. com/watch? v=d 0 z. ION 8 xjb. M https: //www. youtube. com/watch? v=z. GM-w. SKFBpo https: //www. youtube. com/watch? v=z. UDDi. Wt. Ft. EM

Molecules

Molecules • Are made up of atoms bonded together. • The structure of an individual atom determines: –Whether the atom can form bonds. –How many other atoms it can bond to.

Molecules • A molecule is the smallest particle of a substance that exists alone. • This is a picture of a water molecule. It is two parts hydrogen and one part oxygen.

Molecules Na. Cl, salt Molecule: The smallest identifiable unit that retains the physical and chemical properties of the pure substances. Ethanol, C 2 H 6 O Buckyball, C 60

Diatomic Molecules Are molecules of two atoms of the same element. Elements found is nature as diatomic are, H 2, O 2, N 2, F 2, Cl 2, Br 2 and I 2.

https: //www. youtube. com/watch? v=H 57 ZUl. BQXGM Compounds

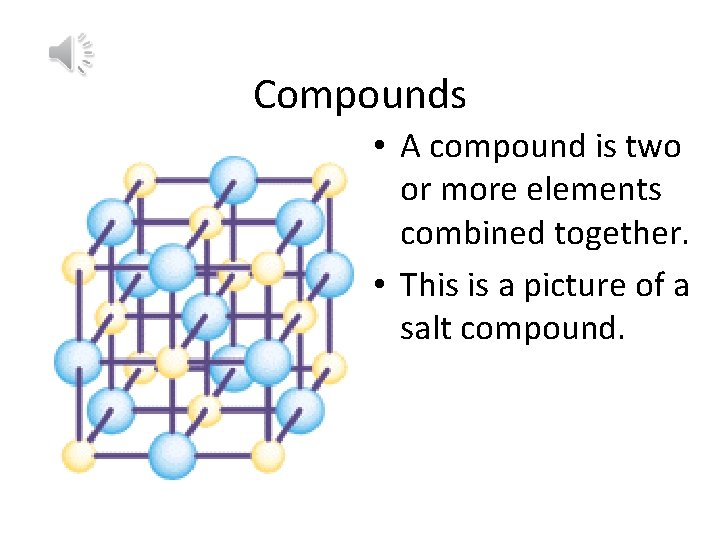
Compounds • A compound is two or more elements combined together. • This is a picture of a salt compound.

Compounds • Is a combination of 2 or more elements in definite ratios by mass. • The character of each element is lost when forming a compound (e. g. , think of Na. Cl).

Compounds • A compound is a substance whose smallest unit is made up of atoms of more than one element bonded together. • Compounds often have properties that are different from the elements that make them up. • Examples: Water, salt, sugar

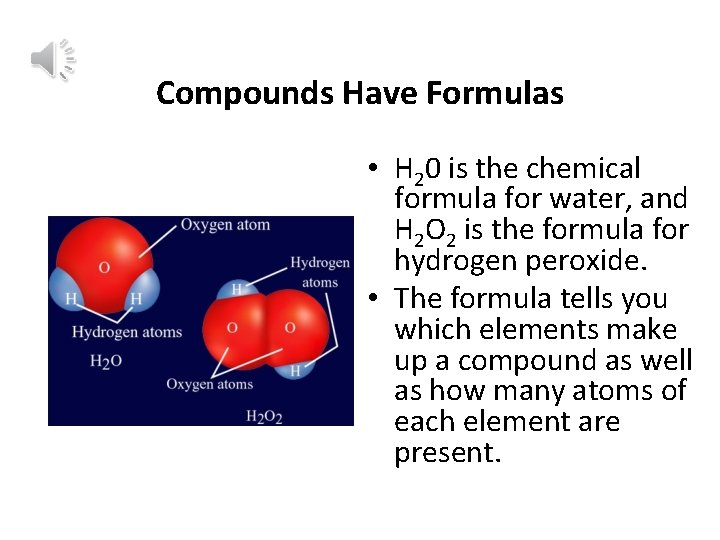
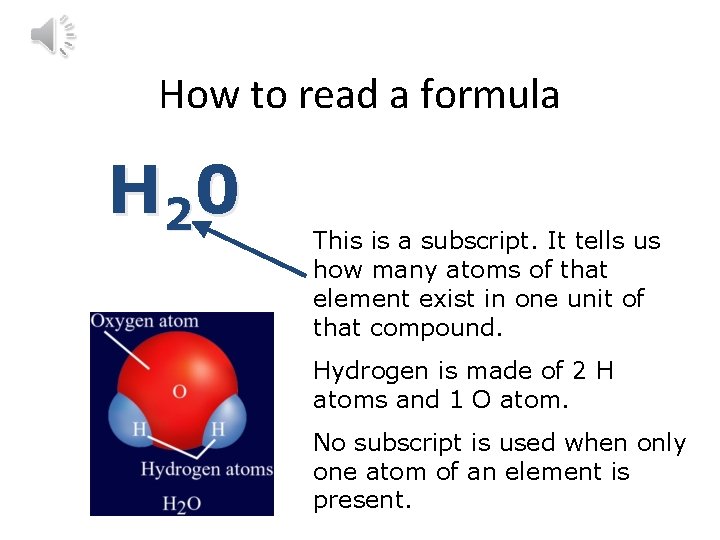
Compounds Have Formulas • H 20 is the chemical formula for water, and H 2 O 2 is the formula for hydrogen peroxide. • The formula tells you which elements make up a compound as well as how many atoms of each element are present.

How to read a formula H 2 0 This is a subscript. It tells us how many atoms of that element exist in one unit of that compound. Hydrogen is made of 2 H atoms and 1 O atom. No subscript is used when only one atom of an element is present.

Let’s try it… • Using your white board tell how many atoms there are in each element. • Sulfuric Acid H 2 SO 4 – 2 Hydrogen – 4 Oxygen • Hydrogen Peroxide H 2 O 2 – 2 Hydrogen – 2 Oxygen