Atoms Elements Molecules and compounds Objectives q Definition
Atoms , Elements , Molecules and compounds Objectives: q Definition of atoms, elements, molecules and compounds. q Identifying the atoms, elements, molecules and compounds.

Atoms
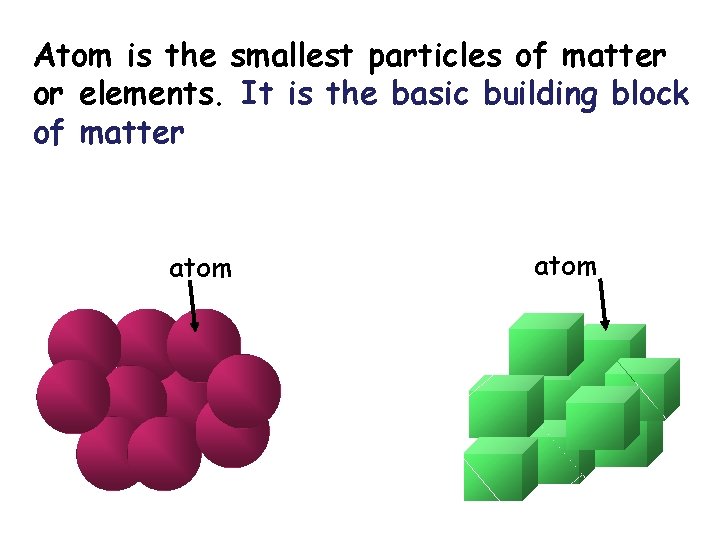
Atom is the smallest particles of matter or elements. It is the basic building block of matter atom
Elements
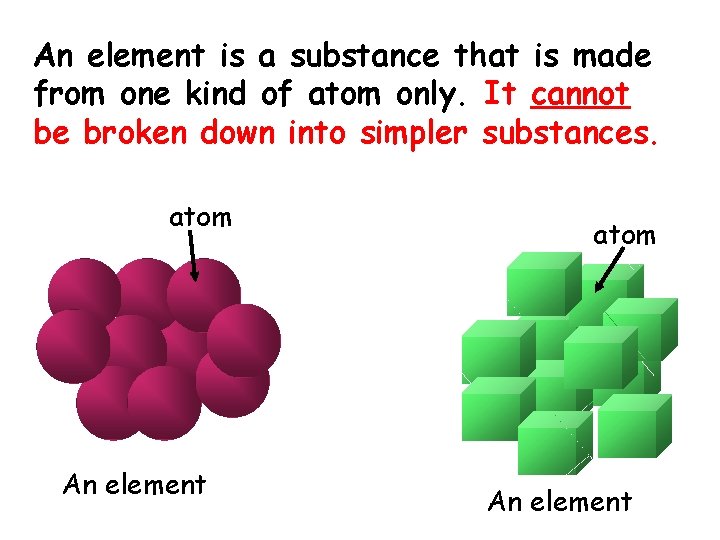
An element is a substance that is made from one kind of atom only. It cannot be broken down into simpler substances. atom An element

Compounds
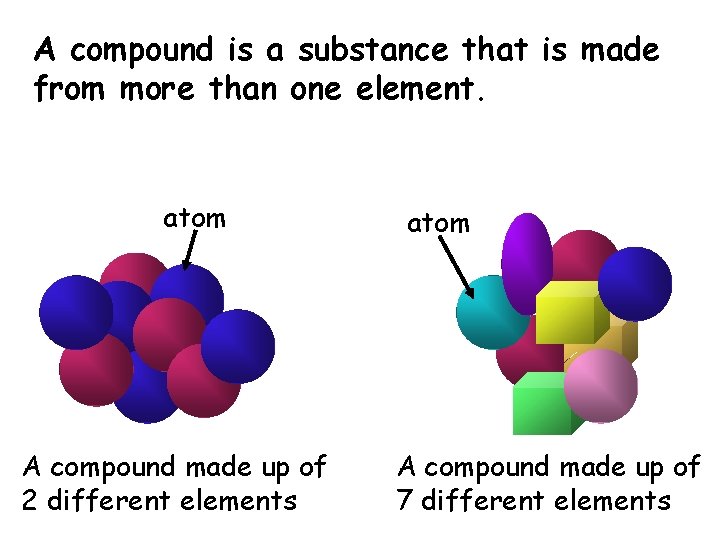
A compound is a substance that is made from more than one element. atom A compound made up of 2 different elements atom A compound made up of 7 different elements
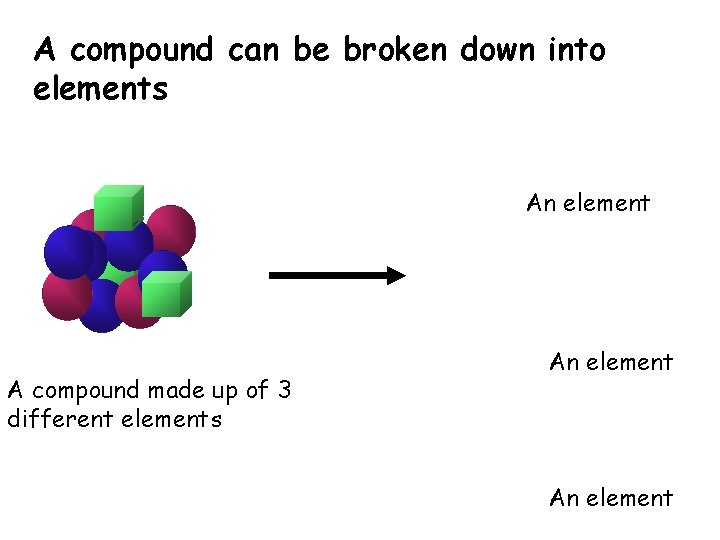
A compound can be broken down into elements An element A compound made up of 3 different elements An element
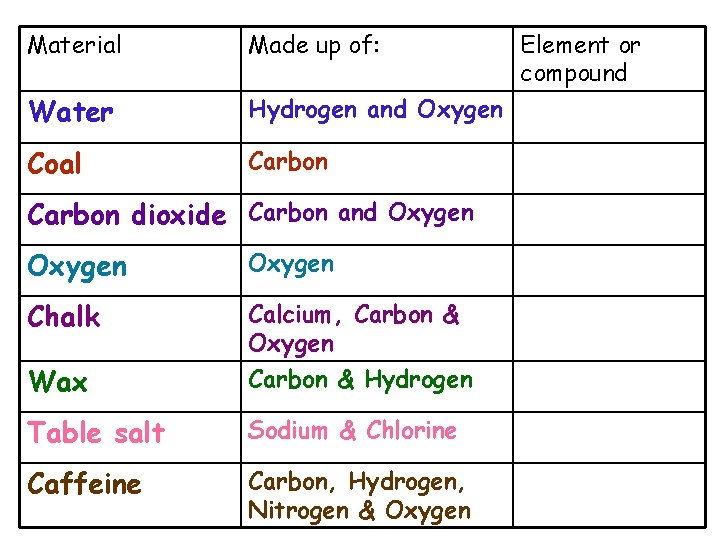
Material Made up of: Water Hydrogen and Oxygen Coal Carbon dioxide Carbon and Oxygen Chalk Wax Calcium, Carbon & Oxygen Carbon & Hydrogen Table salt Sodium & Chlorine Caffeine Carbon, Hydrogen, Nitrogen & Oxygen Element or compound
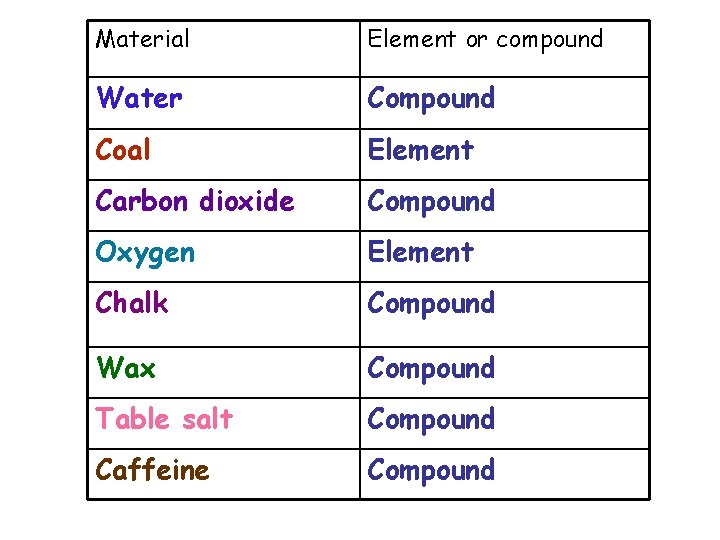
Material Element or compound Water Compound Coal Element Carbon dioxide Compound Oxygen Element Chalk Compound Wax Compound Table salt Compound Caffeine Compound

Molecules
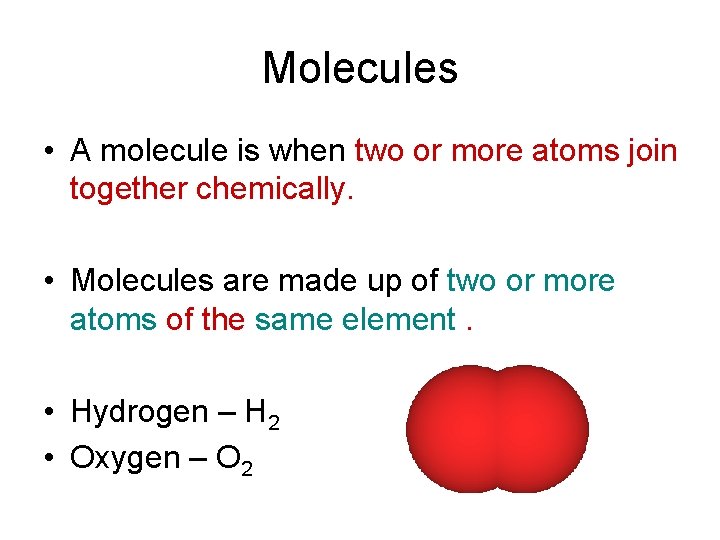
Molecules • A molecule is when two or more atoms join together chemically. • Molecules are made up of two or more atoms of the same element. • Hydrogen – H 2 • Oxygen – O 2
Difference between Molecules and compounds
What is the difference between a compound a molecule? • A molecule is formed when two or more atoms join together chemically. • A compound is formed when two or more elements joint together. • All compounds are molecules but not all molecules are compounds.
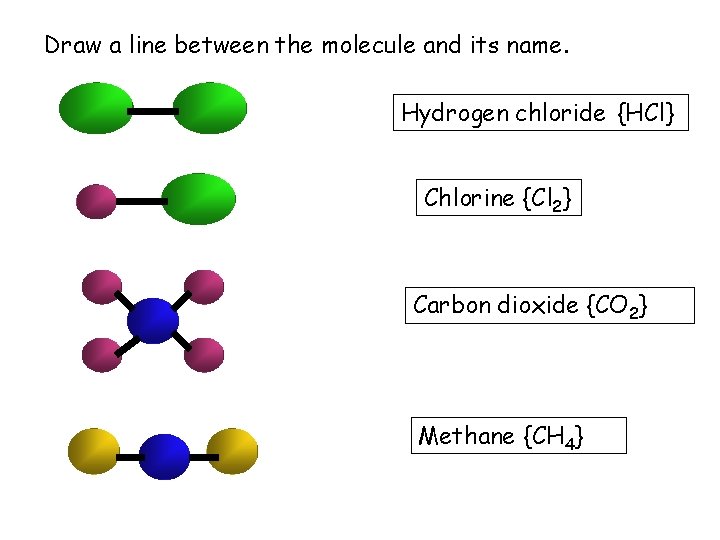
Draw a line between the molecule and its name. Hydrogen chloride {HCl} Chlorine {Cl 2} Carbon dioxide {CO 2} Methane {CH 4}
- Slides: 15