Atoms Elements Chapter Outline Development of Atomic Theories













- Slides: 59

Atoms & Elements

Chapter Outline • Development of Atomic Theories • The composition of Atom • Chemical properties of Atoms, the Periodicity and Periodic Table • Isotopes 2

Experiencing Atoms • Atoms: incredibly small, yet compose everything • atoms are the pieces of Elements • properties of the atoms determine the properties of the elements 3

Experiencing Atoms • 91 elements found in nature • Over 20 we have made in laboratories, and scientists are going to make more • Each element has its own, unique kind of atoms üdifferent structures üdifferent properties 4

Dalton’s Atomic Theory 1. Elements are composed of atoms 2. All atoms of an element are identical 3. Atoms combine in simple, wholenumber ratios to form molecules of compounds John Dalton (1766 -1844) CO 2 (O=C=O): 2 Oxygen atom + 1 Carbon atom H 2 O (H-O-H): 2 Hydrogen atom + 1 Oxygen atom 4. In chemical reactions, atoms are not broken or changed into another type 2 H 2 + O 2 2 H 2 O 5

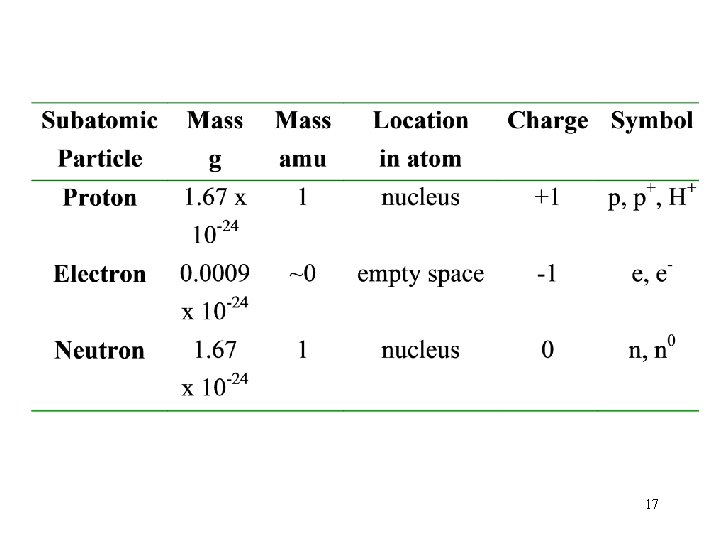
The Size of Atoms Atomic Mass Unit (amu): 1 amu = 1. 66 10 -24 g Hydrogen the smallest atom ümass of H atom= 1. 67 x 10 -24 g ~ 1 amu üvolume of H atom = 2. 1 x 10 -25 cm 3 6

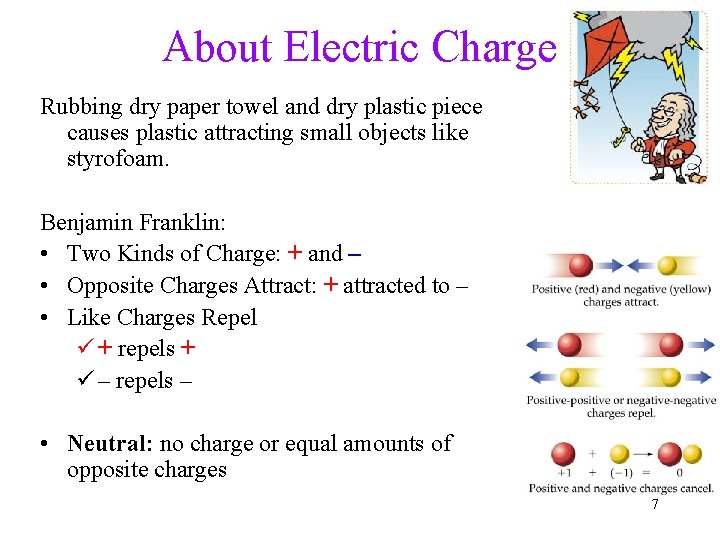
About Electric Charge Rubbing dry paper towel and dry plastic piece causes plastic attracting small objects like styrofoam. Benjamin Franklin: • Two Kinds of Charge: + and – • Opposite Charges Attract: + attracted to – • Like Charges Repel ü + repels + ü – repels – • Neutral: no charge or equal amounts of opposite charges 7

Lightning: Neutralization of Charges accumulated between the clouds and the ground 8

1900 s: The Atom is Divisible! Discovery of Electrons (J. J. Thomson et al. ): • The atom had pieces called electrons • Electrons are much smaller than atoms and carry a “ -” charge üthe mass of the electron is 1/1836 th the mass of a hydrogen atom üthe charge on the electron is the fundamental unit of charge which we will call – 1 charge units 9

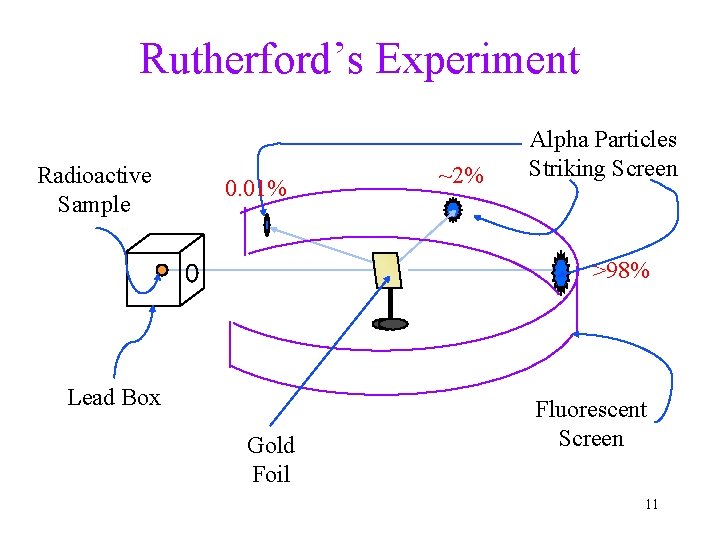
What is Inside an atom? https: //www. youtube. com/watch? v=TKt 3 Vu. MMH_E (7 -10 min) Ernest Rutherford’s experiment (1909): bombardment of a sheet of large atoms (as target) with small, high energy particles • bullet = alpha particles, target atoms = gold foil ü a particles have a mass of 4 amu & charge of +2 c. u. ü gold has a mass of 197 amu & is very malleable 10

Rutherford’s Experiment Radioactive Sample 0. 01% ~2% Alpha Particles Striking Screen >98% Lead Box Gold Foil Fluorescent Screen 11

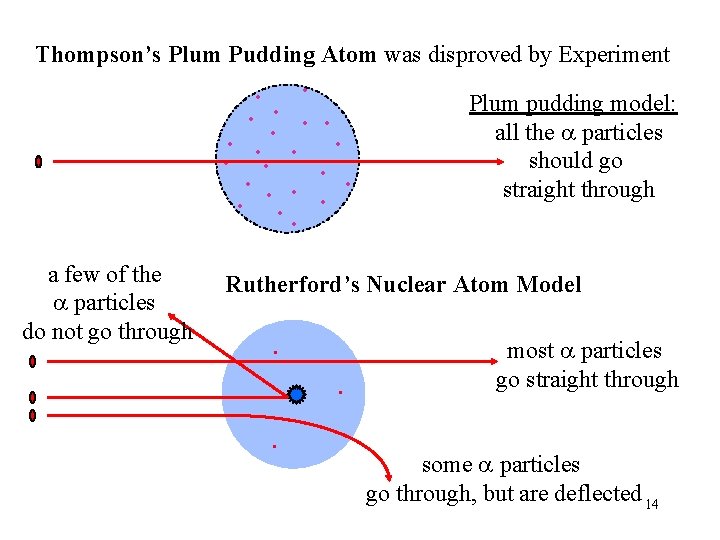
Rutherford’s Results Thompson’s model of atom predicts there is no heavy mass or high charge within the atom, a particles should penetrate without obstruction • > 98% of the a particles: went straight through • ~2% of the a particles went through but were deflected by large angles • ~0. 01% of the a particles bounced off the gold foil: “. . . as if you fired a 15” canon shell at a piece of tissue paper and it came back and hit you. ” 12

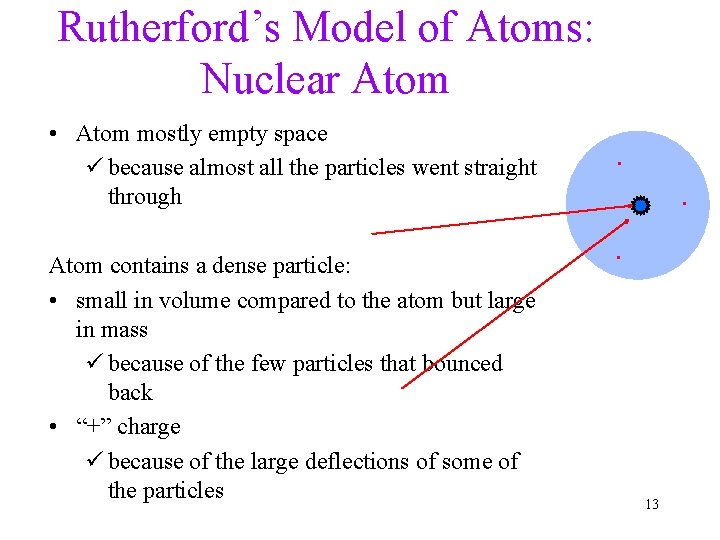
Rutherford’s Model of Atoms: Nuclear Atom • Atom mostly empty space ü because almost all the particles went straight through Atom contains a dense particle: • small in volume compared to the atom but large in mass ü because of the few particles that bounced back • “+” charge ü because of the large deflections of some of the particles . . . 13

Thompson’s Plum Pudding Atom was disproved by Experiment • • • a few of the a particles do not go through • • • Plum pudding model: all the a particles should go straight through • Rutherford’s Nuclear Atom Model . . . most a particles go straight through some a particles go through, but are deflected 14

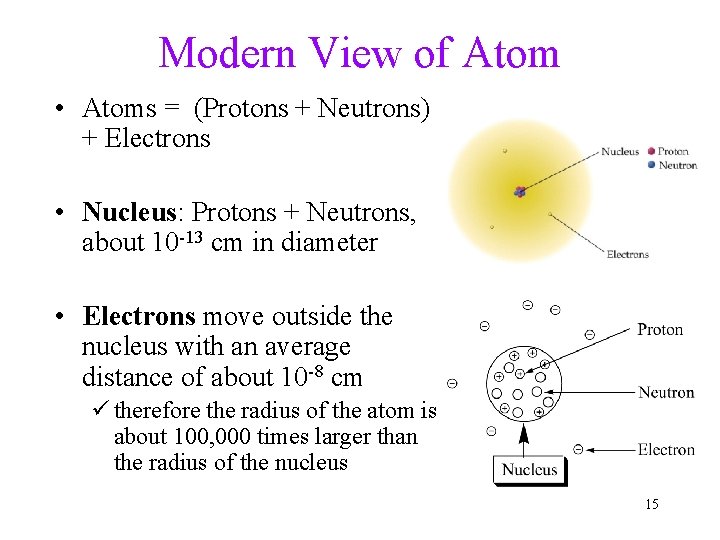
Modern View of Atom • Atoms = (Protons + Neutrons) + Electrons • Nucleus: Protons + Neutrons, about 10 -13 cm in diameter • Electrons move outside the nucleus with an average distance of about 10 -8 cm ü therefore the radius of the atom is about 100, 000 times larger than the radius of the nucleus 15

Inside the Nucleus: Neutron and Protons • Protons: “+” charge and a mass of 1 amu üthe number of proton equals the number of electrons in an atom (electrically neutral) • Neutron: have no charge and a mass of 1 amu üthe masses of the proton and neutron are both approximately 1 amu 16

17

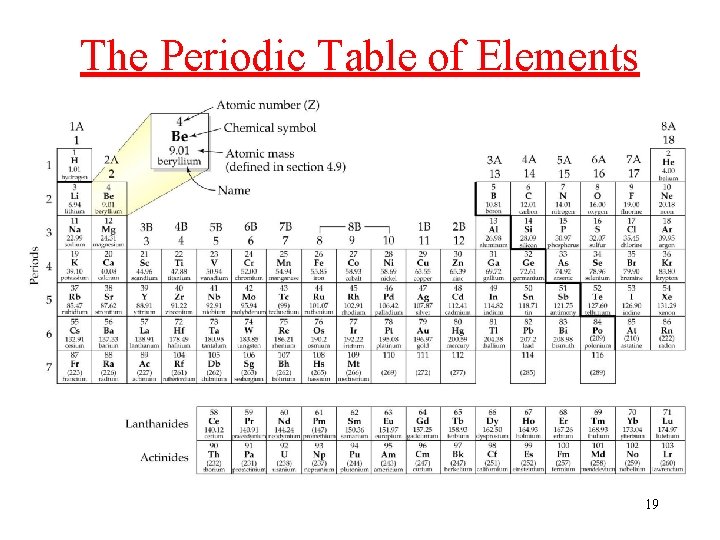
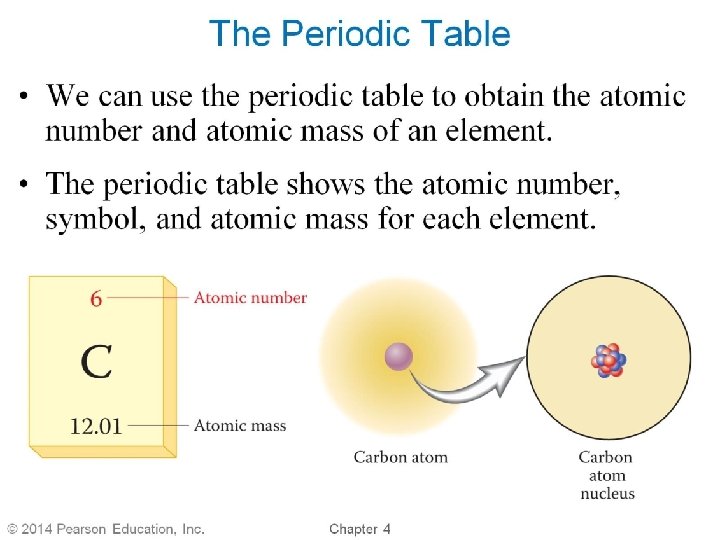
Elements • each Element has a unique number of protons in its nucleus • Atomic number: the number of Protons in the nucleus of an atom üthe elements are arranged on the Periodic Table in order of their atomic numbers • each element has a unique name and symbol üsymbol either one or two letters Øone capital letter or one capital letter + one lower case 18

The Periodic Table of Elements 19

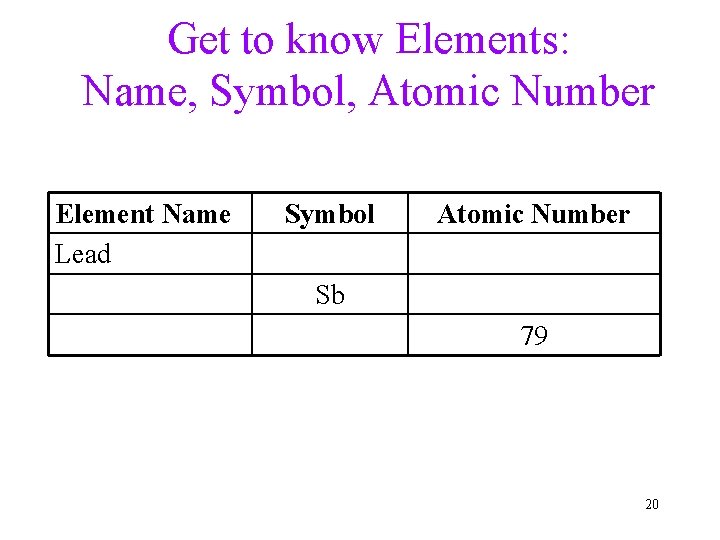
Get to know Elements: Name, Symbol, Atomic Number Element Name Lead Symbol Atomic Number Sb 79 20

Mendeleev: Periodicity • order elements by atomic mass repeating pattern of properties • Periodic Law – When the elements are arranged in order of increasing relative mass, certain sets of properties recur periodically • used pattern to predict properties of undiscovered elements • A good documentary: Mendeleev (in the first 25 minutes, 20 -25 min as my fav ) https: //www. youtube. com/watch? v=y. G 0 G_Nkw. OMc 21

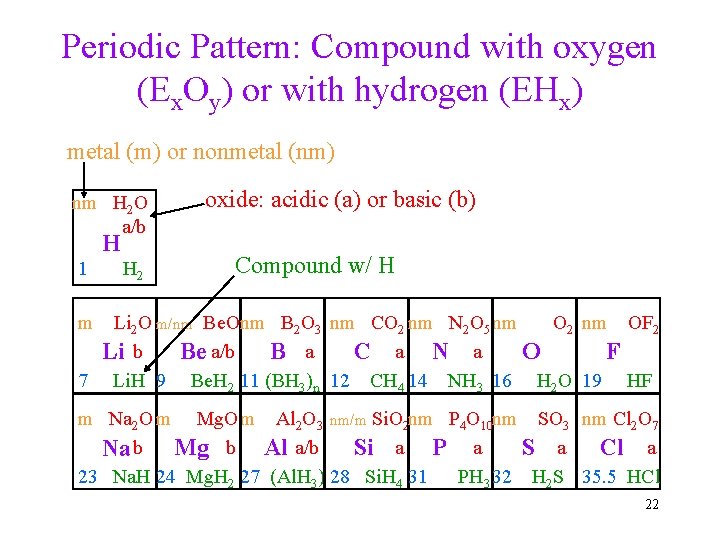
Periodic Pattern: Compound with oxygen (Ex. Oy) or with hydrogen (EHx) metal (m) or nonmetal (nm) nm H 2 O a/b H 1 H 2 oxide: acidic (a) or basic (b) Compound w/ H O 2 nm OF 2 Li 2 O m/nm Be. Onm B 2 O 3 nm CO 2 nm N 2 O 5 nm Li b Be a/b B a C a N a O F 7 Li. H 9 Be. H 2 11 (BH 3)n 12 CH 4 14 NH 3 16 H 2 O 19 HF m m Na 2 O m Mg. O m Al 2 O 3 nm/m Si. O 2 nm P 4 O 10 nm SO 3 nm Cl 2 O 7 S a Cl a Na b Mg b Al a/b Si a P a 23 Na. H 24 Mg. H 2 27 (Al. H 3) 28 Si. H 4 31 PH 3 32 H 2 S 35. 5 HCl 22

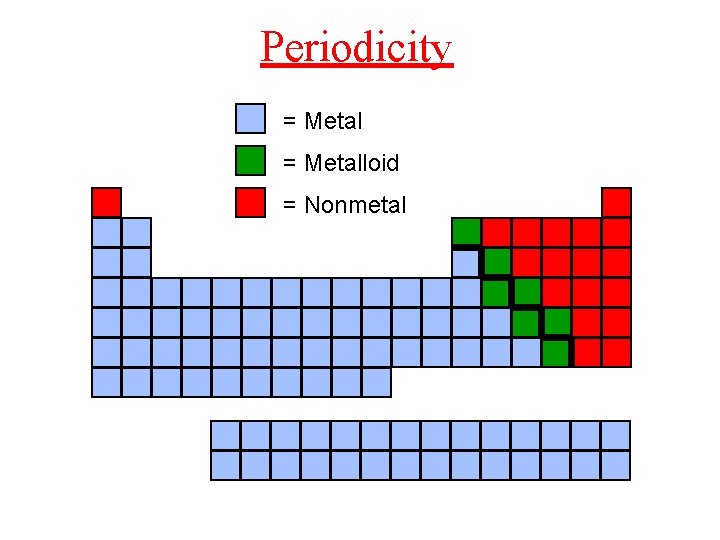
Periodicity = Metalloid = Nonmetal

Metals: Physical vs. Chemical Properties • solids at room temperature, except Hg • reflective surface ü shiny • conduct heat, electricity • Malleable (can be shaped) • Tend to Lose electrons and form Cations in reactions. Na Na+ + e • about 75% of the elements are metals • lower left on the table 24

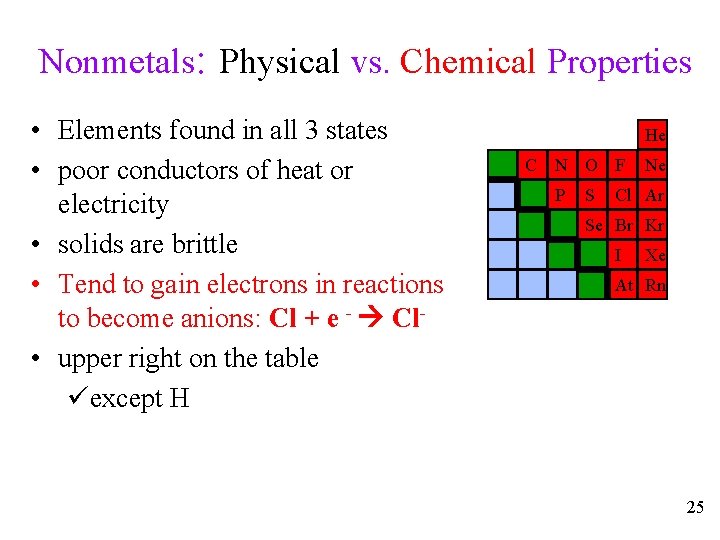
Nonmetals: Physical vs. Chemical Properties • Elements found in all 3 states • poor conductors of heat or electricity • solids are brittle • Tend to gain electrons in reactions to become anions: Cl + e - Cl • upper right on the table üexcept H He C N O F P S Ne Cl Ar Se Br Kr I Xe At Rn 25

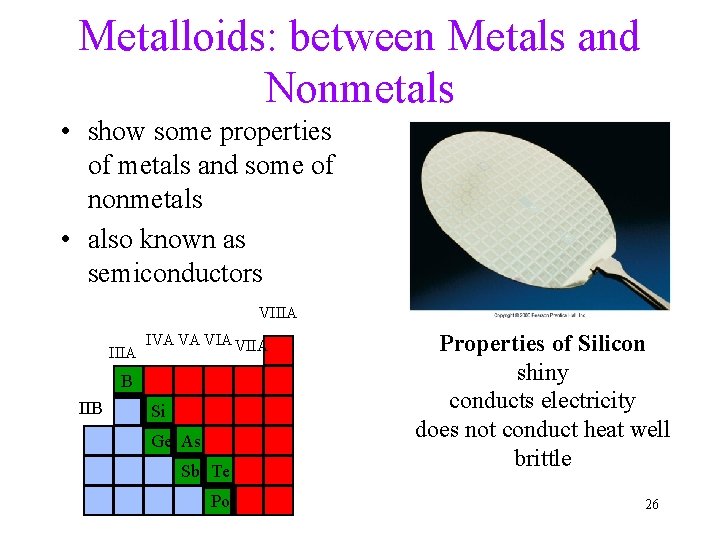
Metalloids: between Metals and Nonmetals • show some properties of metals and some of nonmetals • also known as semiconductors VIIIA IVA VA VIIA B IIB Si Ge As Sb Te Po Properties of Silicon shiny conducts electricity does not conduct heat well brittle 26

The Modern Periodic Table • Elements with similar chemical and physical properties are in the same column • Columns are called _____ or Families üdesignated by a number and letter at top • Rows are _______s • each period shows the pattern of properties repeated in the next period Group period 27

The Modern Periodic Table • Main Group = Representative Elements = ‘A’ Groups IA~VIIIA • Transition Elements = ‘B’ groups: IB~VIIIB üall metals • Bottom rows = Inner Transition Elements = Rare Earth Elements ümetals üreally belong in Period 6 & 7 28

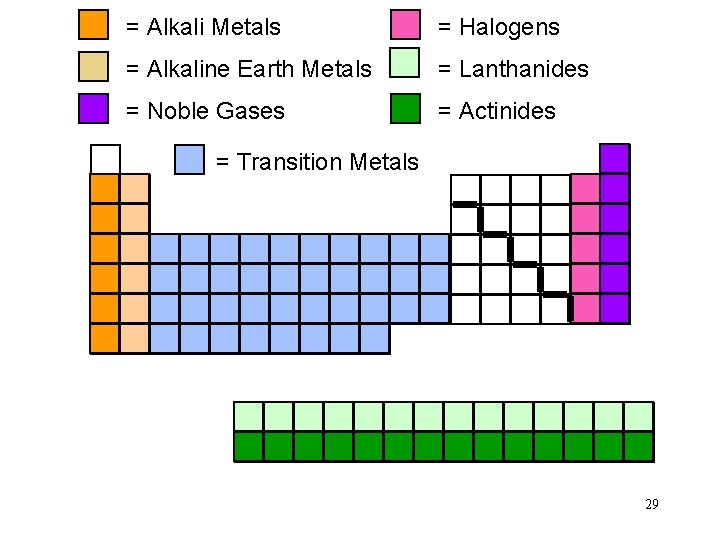
= Alkali Metals = Halogens = Alkaline Earth Metals = Lanthanides = Noble Gases = Actinides = Transition Metals 29

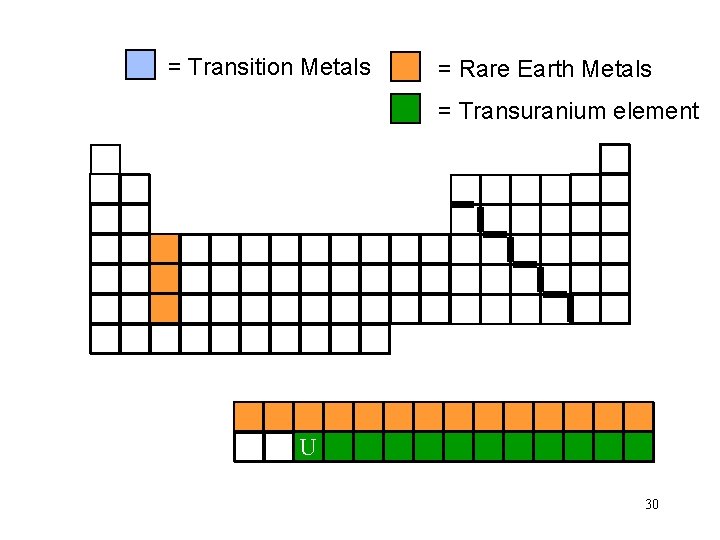
= Transition Metals = Rare Earth Metals = Transuranium element U 30

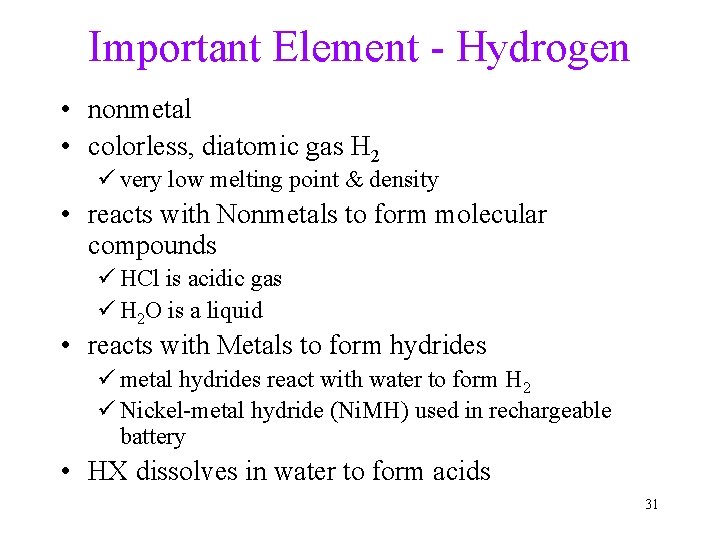
Important Element - Hydrogen • nonmetal • colorless, diatomic gas H 2 ü very low melting point & density • reacts with Nonmetals to form molecular compounds ü HCl is acidic gas ü H 2 O is a liquid • reacts with Metals to form hydrides ü metal hydrides react with water to form H 2 ü Nickel-metal hydride (Ni. MH) used in rechargeable battery • HX dissolves in water to form acids 31

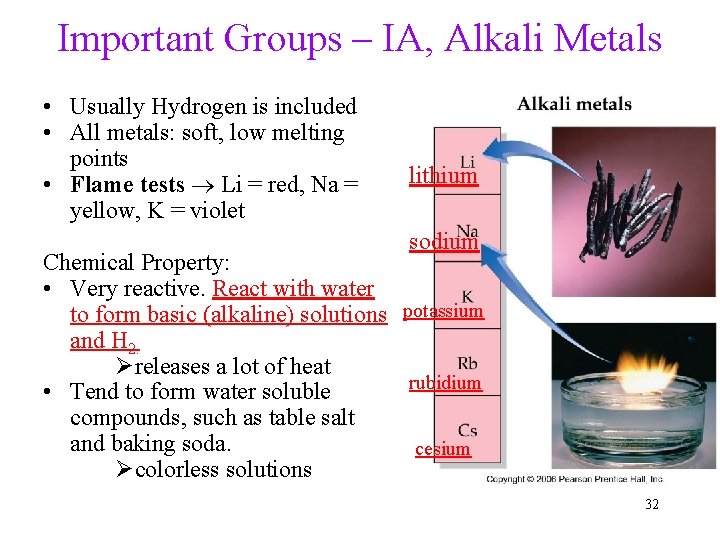
Important Groups – IA, Alkali Metals • Usually Hydrogen is included • All metals: soft, low melting points • Flame tests Li = red, Na = yellow, K = violet lithium sodium Chemical Property: • Very reactive. React with water to form basic (alkaline) solutions potassium and H 2. Øreleases a lot of heat rubidium • Tend to form water soluble compounds, such as table salt and baking soda. cesium Øcolorless solutions 32

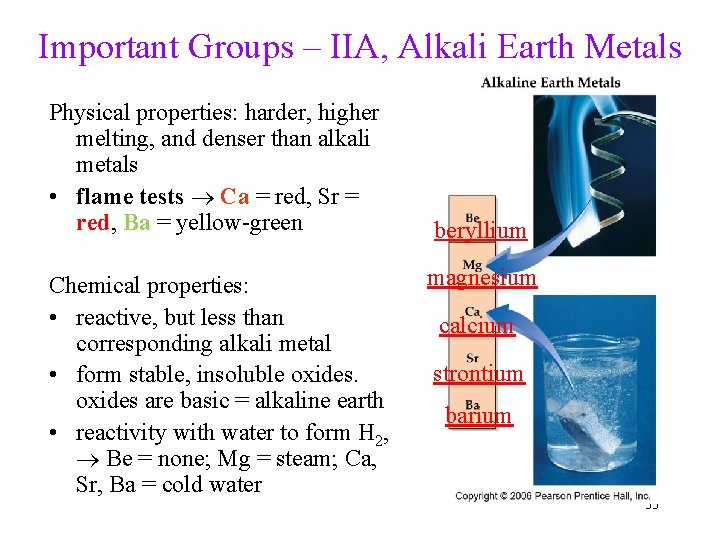
Important Groups – IIA, Alkali Earth Metals Physical properties: harder, higher melting, and denser than alkali metals • flame tests Ca = red, Sr = red, Ba = yellow-green Chemical properties: • reactive, but less than corresponding alkali metal • form stable, insoluble oxides are basic = alkaline earth • reactivity with water to form H 2, Be = none; Mg = steam; Ca, Sr, Ba = cold water beryllium magnesium calcium strontium barium 33

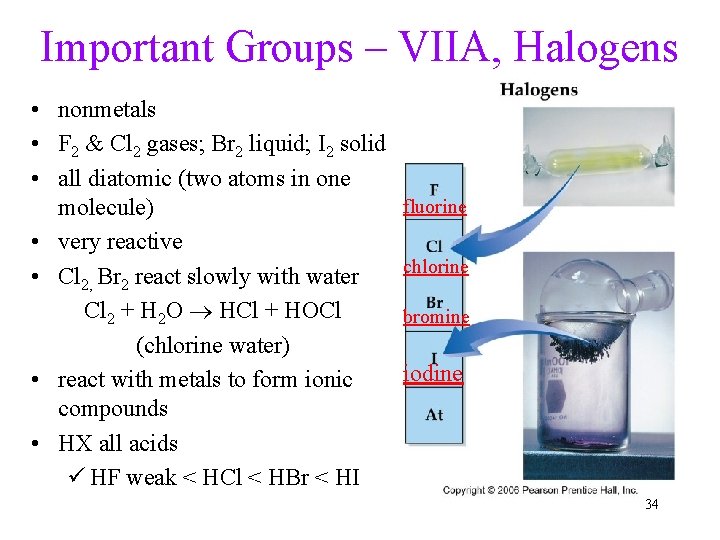
Important Groups – VIIA, Halogens • nonmetals • F 2 & Cl 2 gases; Br 2 liquid; I 2 solid • all diatomic (two atoms in one molecule) • very reactive • Cl 2, Br 2 react slowly with water Cl 2 + H 2 O HCl + HOCl (chlorine water) • react with metals to form ionic compounds • HX all acids ü HF weak < HCl < HBr < HI fluorine chlorine bromine iodine 34

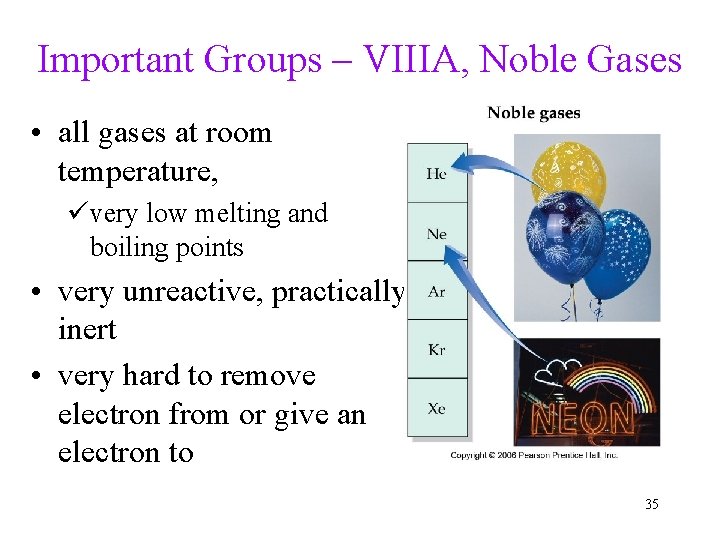
Important Groups – VIIIA, Noble Gases • all gases at room temperature, üvery low melting and boiling points • very unreactive, practically inert • very hard to remove electron from or give an electron to 35

Ion: Charged Atom • The number of protons determines the element! üall sodium atoms have 11 protons in the nucleus • In a chemical change, the number of protons in the nucleus of the atom doesn’t change! üno transmutation during a chemical change!! üduring radioactive and nuclear changes, atoms do transmute • Atoms in a compound are often electrically charged, these are called ions 36

Ions • Atoms acquire a charge by gaining or losing electrons ü not protons!! • Ion Charge = # protons – # electrons • ions with a + charge are called cations ü more protons than electrons ü form by losing electrons • ions with a – charge are called anions ü more electrons than protons ü form by gaining electrons 37

Atomic Structures of Cations • Metals form cations • More positive charge, the fewer electrons than the neutral atom üNa atom = 11 p+ and 11 e-, Na+ ion = 11 p+ and 10 eüCa atom = 20 p+ and 20 e-, Ca 2+ ion = 20 p+ and 18 e • Cations are named the same as the metal sodium Na Na+ + 1 esodium ion calcium Ca 2+ + 2 ecalcium ion • The charge on a cation can OFTEN be determined from the Group number on the Periodic Table üGroup 1 A +1, Group 2 A +2, (Al, Ga, In) +3 38

Atomic Structures of Anions • Nonmetals form anions • For each negative charge the ion has 1 more electron than the neutral atom ü F = 9 e-, F- = 10 eü P = 15 e-, P 3 - = 18 e- • Anions are named by changing the ending of the name to -ide fluorine F + 1 e- Ffluoride ion oxygen O + 2 e- O 2 oxide ion • The charge on an anion can be determined from the Group number on the Periodic Table ü Group 7 A -1, Group 6 A -2 39

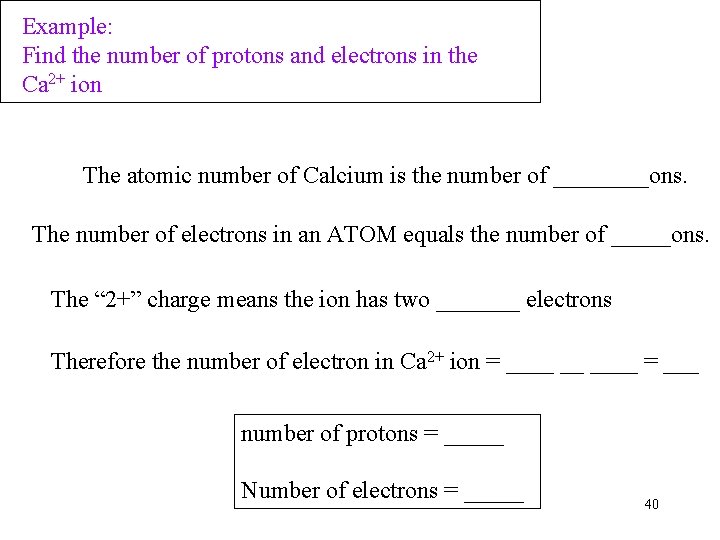
Example: Find the number of protons and electrons in the Ca 2+ ion The atomic number of Calcium is the number of ____ons. The number of electrons in an ATOM equals the number of _____ons. The “ 2+” charge means the ion has two _______ electrons Therefore the number of electron in Ca 2+ ion = ____ __ ____ = ___ number of protons = _____ Number of electrons = _____ 40

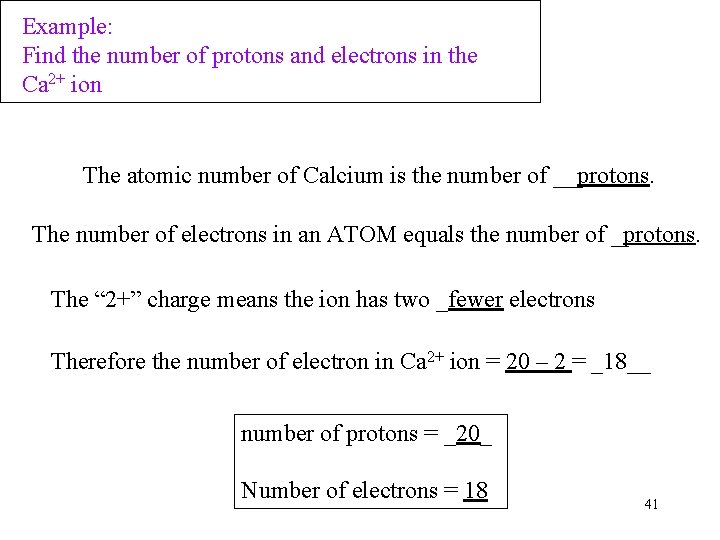
Example: Find the number of protons and electrons in the Ca 2+ ion The atomic number of Calcium is the number of __protons. The number of electrons in an ATOM equals the number of _protons. The “ 2+” charge means the ion has two _fewer electrons Therefore the number of electron in Ca 2+ ion = 20 – 2 = _18__ number of protons = _20_ Number of electrons = 18 41

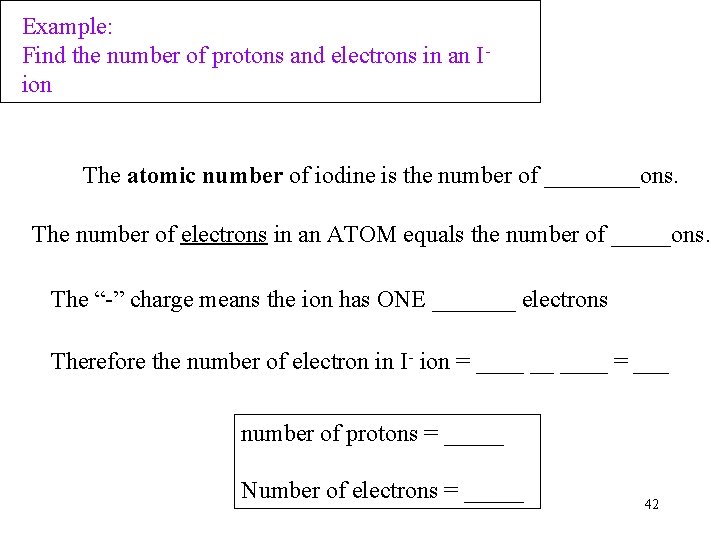
Example: Find the number of protons and electrons in an Iion The atomic number of iodine is the number of ____ons. The number of electrons in an ATOM equals the number of _____ons. The “-” charge means the ion has ONE _______ electrons Therefore the number of electron in I- ion = ____ __ ____ = ___ number of protons = _____ Number of electrons = _____ 42

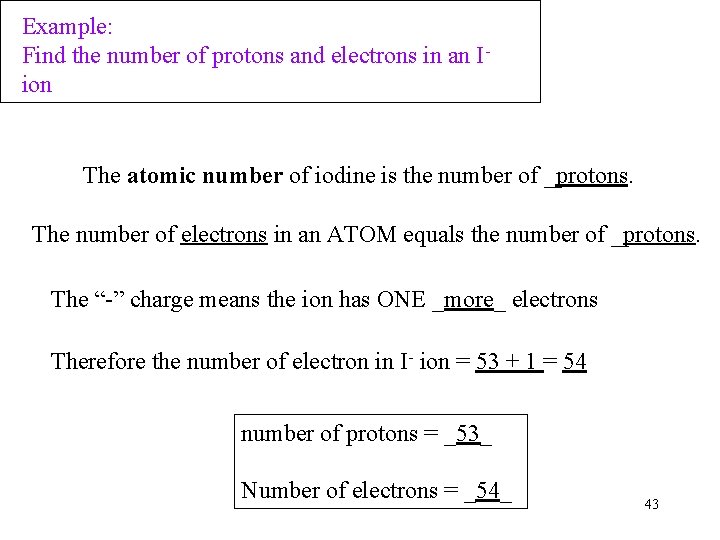
Example: Find the number of protons and electrons in an Iion The atomic number of iodine is the number of _protons. The number of electrons in an ATOM equals the number of _protons. The “-” charge means the ion has ONE _more_ electrons Therefore the number of electron in I- ion = 53 + 1 = 54 number of protons = _53_ Number of electrons = _54_ 43

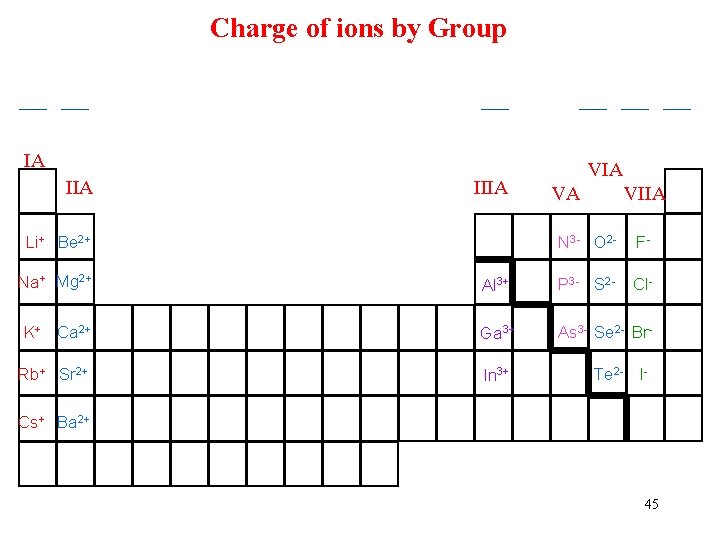
Ion Charge & the Periodic Table • The charge on an ion: an elements position on the Periodic Table • Metals: always positive ions Na+ Ca 2+ Al 3+ • Nonmetals are negative ions Cl- O 2 - N 3 • Main group metals: #charge = #group üMagnesium ion = #Group = ______ • Nonmetals, #ch üPhosphide ion = #group – 8 = _____ 44

Charge of ions by Group __ __ __ IA IIIA Li+ Be 2+ VIA VA VIIA N 3 - O 2 - FCl- Na+ Mg 2+ Al 3+ P 3 - S 2 - K+ Ca 2+ Ga 3+ As 3 - Se 2 - Br- Rb+ Sr 2+ In 3+ Te 2 - I- Cs+ Ba 2+ 45

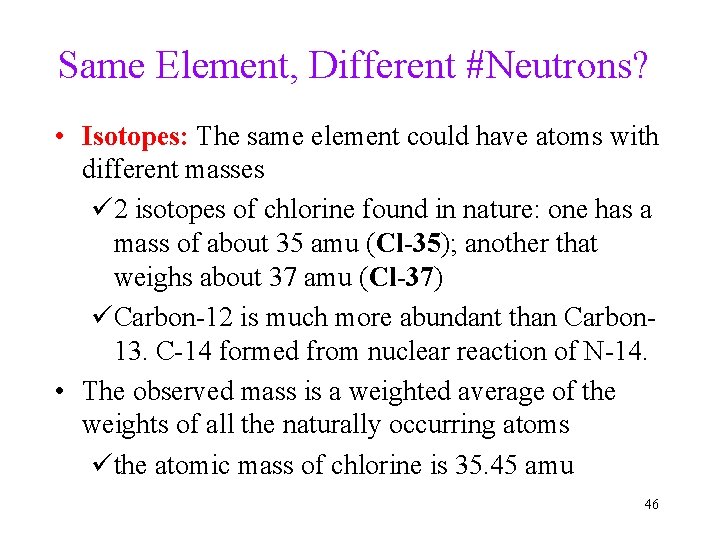
Same Element, Different #Neutrons? • Isotopes: The same element could have atoms with different masses ü 2 isotopes of chlorine found in nature: one has a mass of about 35 amu (Cl-35); another that weighs about 37 amu (Cl-37) üCarbon-12 is much more abundant than Carbon 13. C-14 formed from nuclear reaction of N-14. • The observed mass is a weighted average of the weights of all the naturally occurring atoms üthe atomic mass of chlorine is 35. 45 amu 46

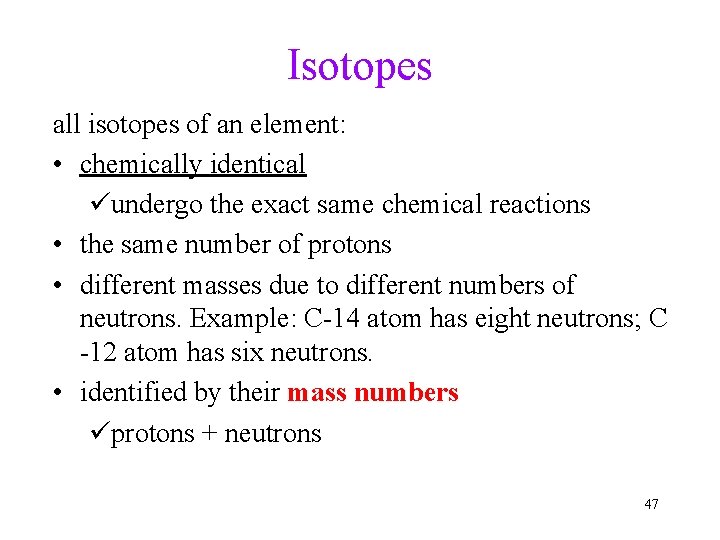
Isotopes all isotopes of an element: • chemically identical üundergo the exact same chemical reactions • the same number of protons • different masses due to different numbers of neutrons. Example: C-14 atom has eight neutrons; C -12 atom has six neutrons. • identified by their mass numbers üprotons + neutrons 47

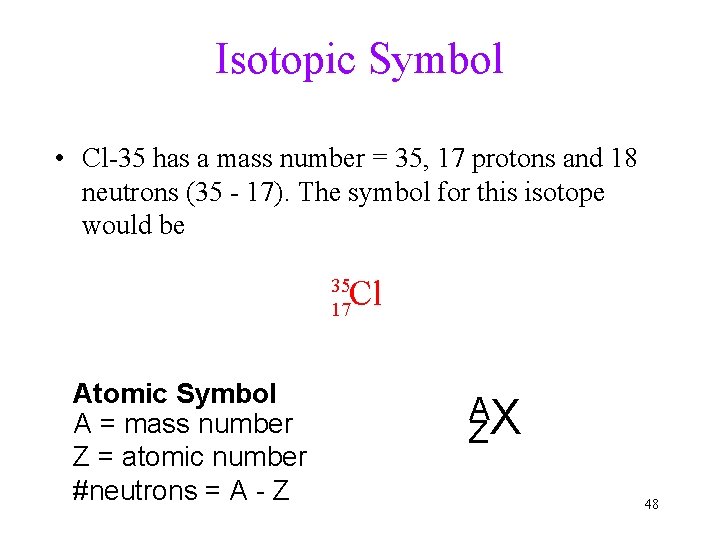
Isotopic Symbol • Cl-35 has a mass number = 35, 17 protons and 18 neutrons (35 - 17). The symbol for this isotope would be Cl 35 17 Atomic Symbol A = mass number Z = atomic number #neutrons = A - Z AX Z 48

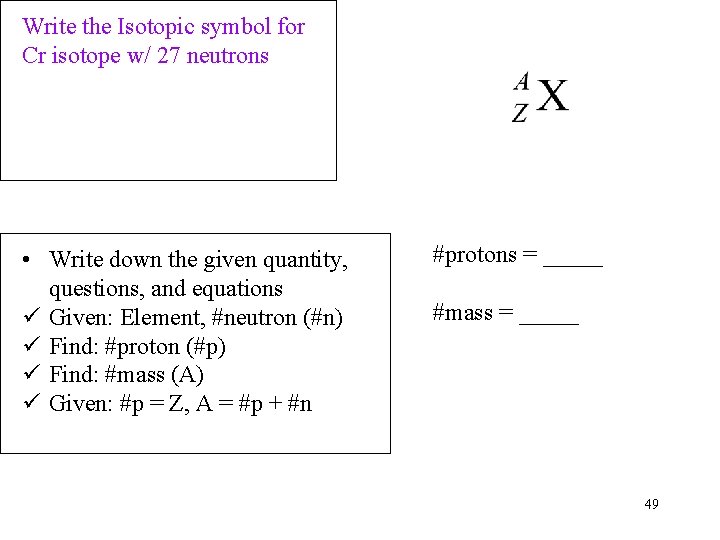
Write the Isotopic symbol for Cr isotope w/ 27 neutrons • Write down the given quantity, questions, and equations ü Given: Element, #neutron (#n) ü Find: #proton (#p) ü Find: #mass (A) ü Given: #p = Z, A = #p + #n #protons = _____ #mass = _____ 49

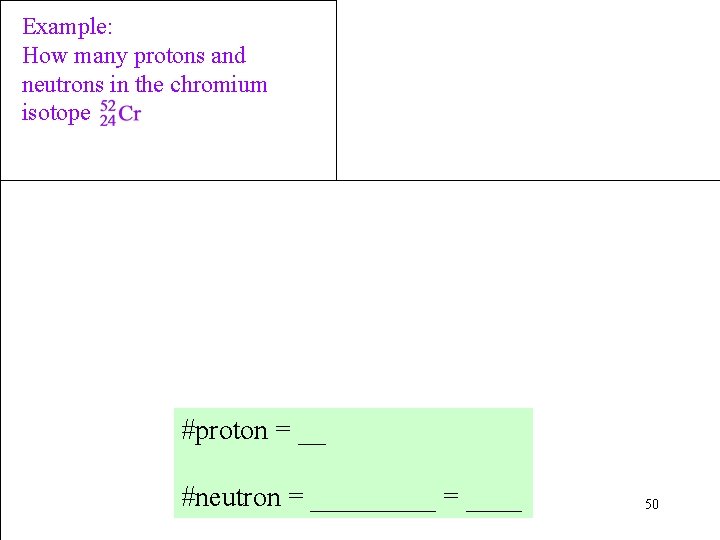
Example: How many protons and neutrons in the chromium isotope #proton = __ #neutron = _____ = ____ 50

Practice - Complete the following table 51

Mass Number is Not the Same as Atomic Mass • the atomic mass is an experimental number determined from all naturally occurring isotopes • the mass number refers to the number of protons + neutrons in one isotope ünatural or man-made 52

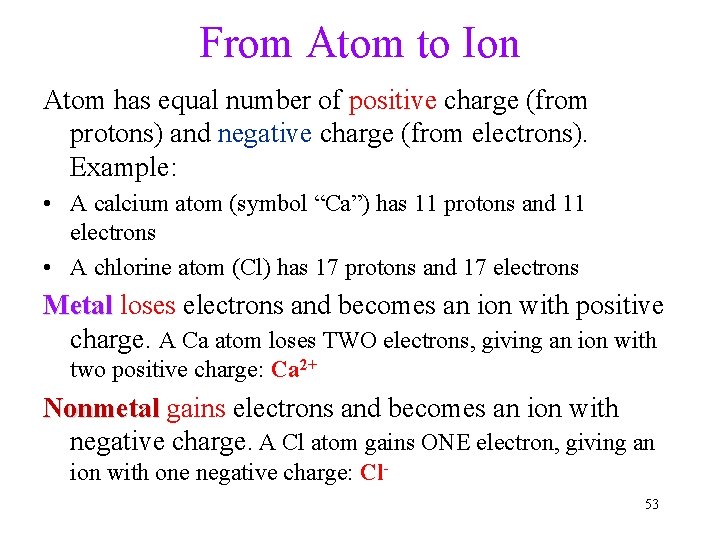
From Atom to Ion Atom has equal number of positive charge (from protons) and negative charge (from electrons). Example: • A calcium atom (symbol “Ca”) has 11 protons and 11 electrons • A chlorine atom (Cl) has 17 protons and 17 electrons Metal loses electrons and becomes an ion with positive charge. A Ca atom loses TWO electrons, giving an ion with two positive charge: Ca 2+ Nonmetal gains electrons and becomes an ion with negative charge. A Cl atom gains ONE electron, giving an ion with one negative charge: Cl 53

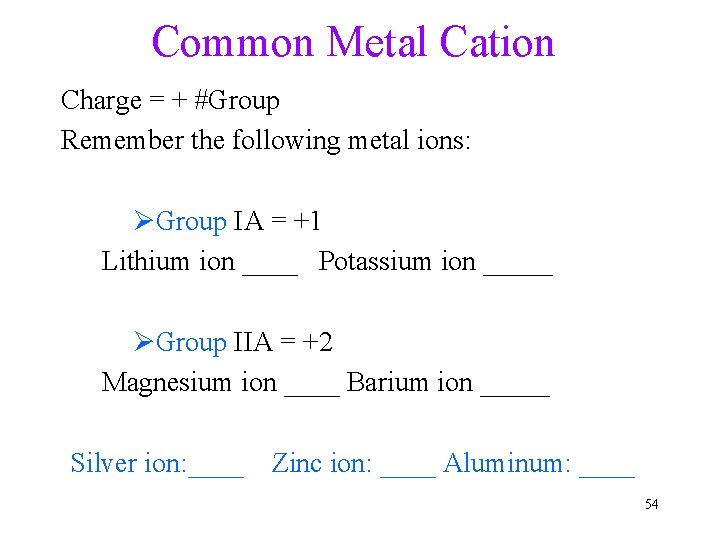
Common Metal Cation Charge = + #Group Remember the following metal ions: ØGroup IA = +1 Lithium ion ____ Potassium ion _____ ØGroup IIA = +2 Magnesium ion ____ Barium ion _____ Silver ion: ____ Zinc ion: ____ Aluminum: ____ 54

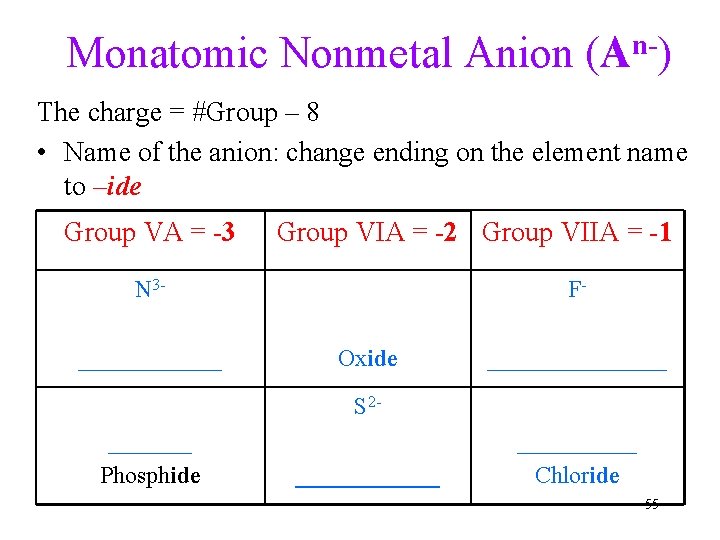
Monatomic Nonmetal Anion (An-) The charge = #Group – 8 • Name of the anion: change ending on the element name to –ide Group VA = -3 Group VIA = -2 Group VIIA = -1 N 3______ FOxide ________ S 2_______ Phosphide ______ Chloride 55


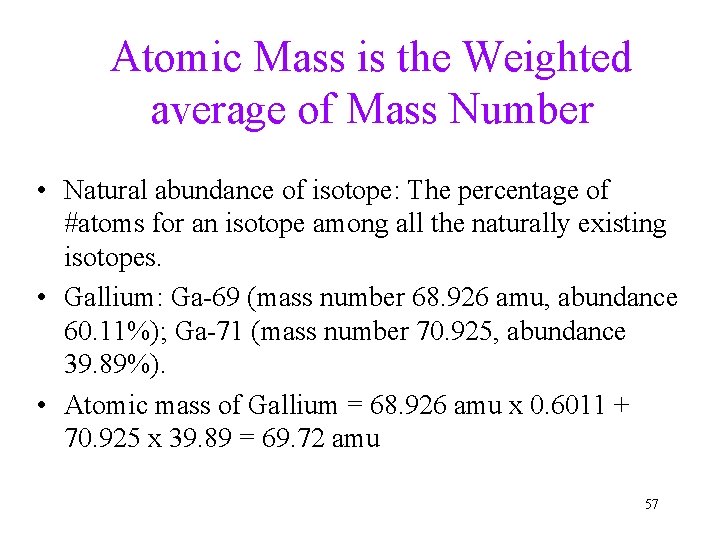
Atomic Mass is the Weighted average of Mass Number • Natural abundance of isotope: The percentage of #atoms for an isotope among all the naturally existing isotopes. • Gallium: Ga-69 (mass number 68. 926 amu, abundance 60. 11%); Ga-71 (mass number 70. 925, abundance 39. 89%). • Atomic mass of Gallium = 68. 926 amu x 0. 6011 + 70. 925 x 39. 89 = 69. 72 amu 57

