Atoms Elements and The Periodic Table I Structure
Atoms, Elements, and The Periodic Table I. Structure of matter A. What is Matter? – Matter is anything that has mass and takes up space (Volume) B. What isn’t Matter? – Light and Heat do not take up space nor do they have a mass.
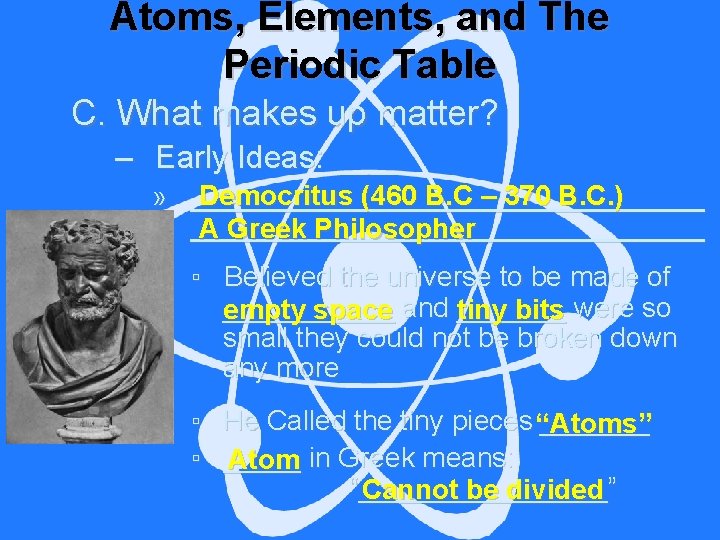
Atoms, Elements, and The Periodic Table C. What makes up matter? – Early Ideas: Democritus (460 B. C – 370 B. C. ) » _________________________________ A Greek Philosopher ▫ Believed the universe to be made of ______ empty space and _______ tiny bits were so small they could not be broken down any more ▫ He Called the tiny pieces “Atoms” _______ ▫ _____ Atom in Greek means: “________” Cannot be divided
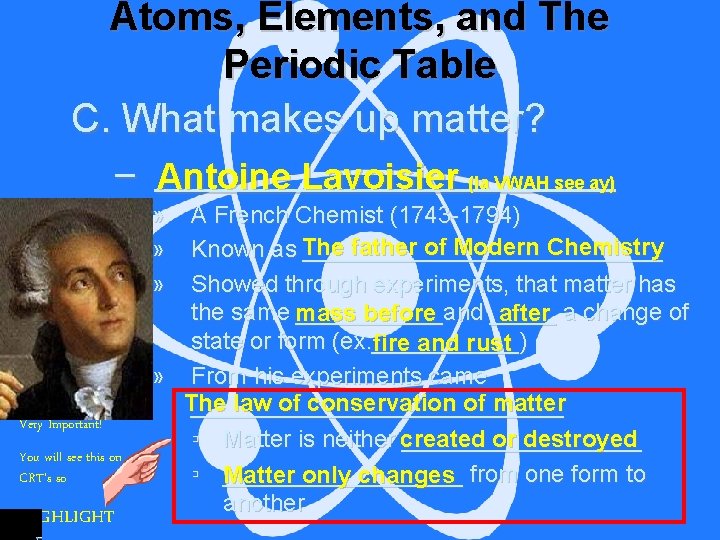
Atoms, Elements, and The Periodic Table C. What makes up matter? – ____________ Antoine Lavoisier (la VWAH see ay) » » » Very Important! You will see this on CRT’s so HIGHLIGHT A French Chemist (1743 -1794) father of Modern Chemistry Known as The ______________ Showed through experiments, that matter has the same mass ______and _____ before after a change of state or form (ex. ______) fire and rust » From his experiments came The law of conservation of matter ______________ or destroyed ▫ Matter is neither created _________ ▫ Matter _________ only changes from one form to another
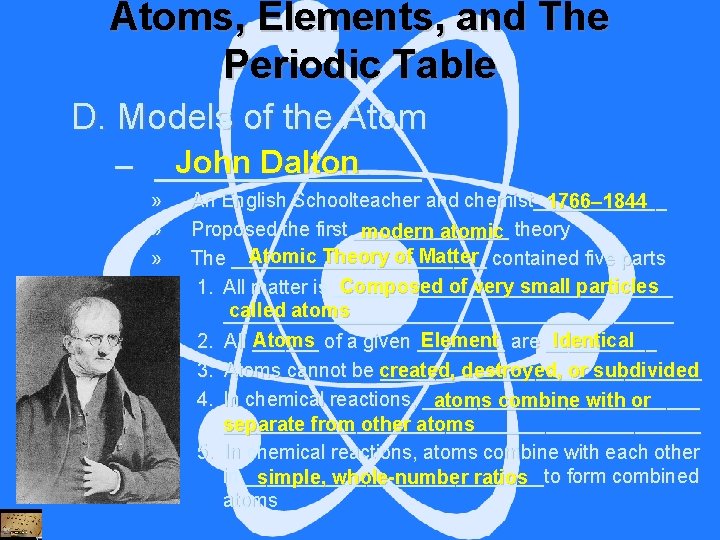
Atoms, Elements, and The Periodic Table D. Models of the Atom John Dalton – ________ » » » An English Schoolteacher and chemist______ 1766– 1844 Proposed the first _______ modern atomic theory Atomic Theory of Matter contained five parts The ____________ Composed of very small particles 1. All matter is _________________ called atoms _______________________ Atoms of a given ____ Element are _____ Identical 2. All ______ 3. Atoms cannot be created, _______________ destroyed, or subdivided 4. In chemical reactions, _____________ atoms combine with or ______________________ separate from other atoms 5. In chemical reactions, atoms combine with each other in ______________to form combined simple, whole-number ratios atoms
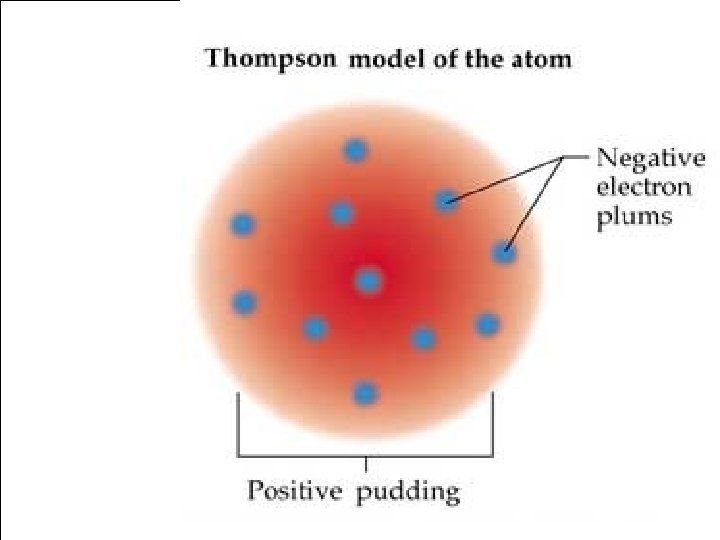
Atoms, Elements, and The Periodic Table D. Models of the Atom – ________ J. J. Thomson » » British Scientist 1856 – 1940 _______________ Conducted experiments using a _________ Cathode Ray Tube » From his experiments he discovered _______ Atoms contained _____________ Negatively Charged Particles ▫ The negative charged particle is called an ____ Electron ◦ An _______ Electron is ______ 1/1, 837 the mass of the Smallest atom, the _____ Hydrogen atom. ◦ Thomson’s model of the atom had electrons spread ball of positive charge Like throughout a __________. Chocolate chips in cookie dough _______________.

Atoms, Elements, and The Periodic Table D. Models of the Atom – ________ Ernest Rutherford » » » New Zealander Physicist An ____________1871 – 1937 Father of Nuclear Physics Known as the ___________ Was a student of _______ J. J. Thomson From experiments with X-Rays, he discovered the atom contained____________. Positive charged particles These were called ____ Protons charge was He also concluded the positive _______ center of the atom The center found in the _________. was called ______ The Nucleus
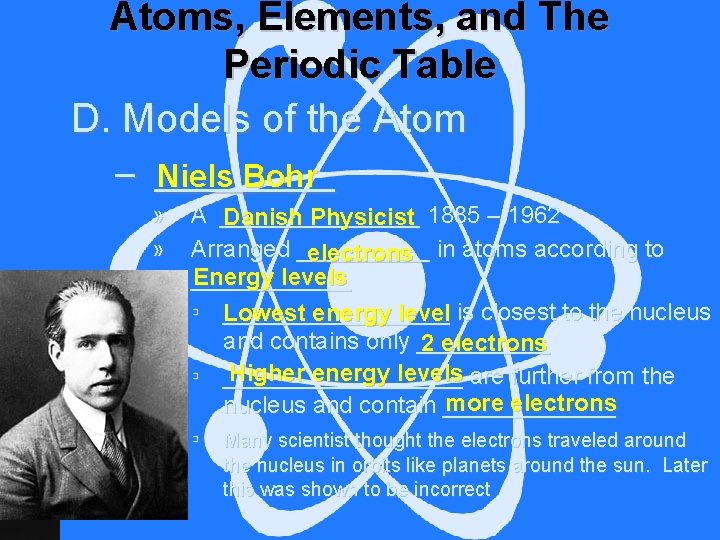
Atoms, Elements, and The Periodic Table D. Models of the Atom – _____ Niels Bohr » » A ________ Danish Physicist 1885 – 1962 Arranged _____ electrons in atoms according to Energy levels ______ ▫ Lowest _________ energy level is closest to the nucleus and contains only _____ 2 electrons Higher energy levels are further from the ▫ _________ more electrons nucleus and contain _______ ▫ Many scientist thought the electrons traveled around the nucleus in orbits like planets around the sun. Later this was shown to be incorrect

- Slides: 8