Atoms Elements and Periodic Table Review What How
Atoms, Elements and Periodic Table

Review � What � How makes up an atom? would you draw a Bohr model of Nitrogen?

Valence Electrons � Valence electrons- the outer most electrons on the Bohr model ◦ Can participate in a chemical bond � How many valence electrons are there in Nitrogen?
Valence Electrons � On a whiteboard, draw a Bohr model for elements atomic numbers 3 -10 � How many valence electrons are in each one?
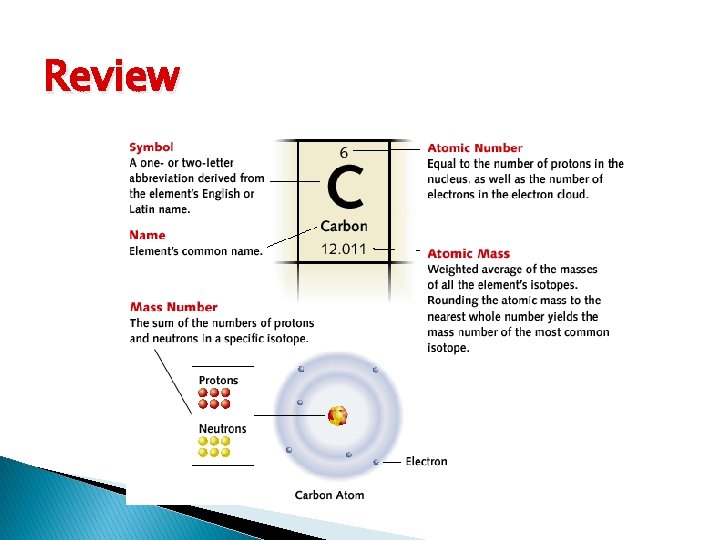
Review
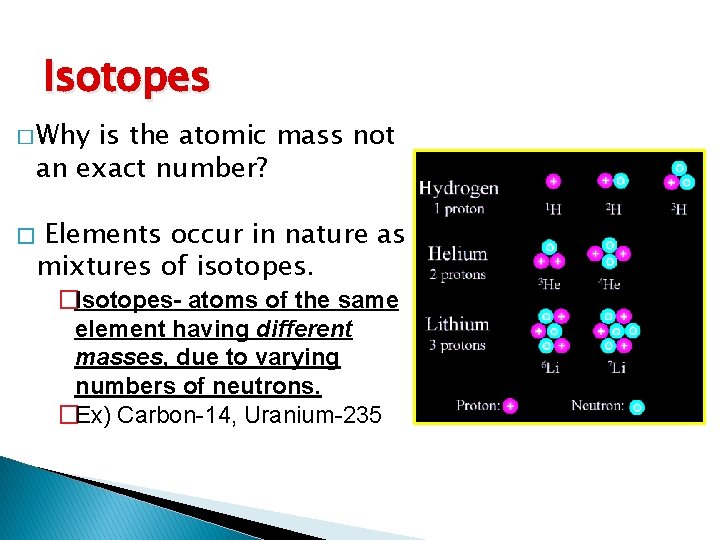
Isotopes � Why is the atomic mass not an exact number? � Elements occur in nature as mixtures of isotopes. �Isotopes- atoms of the same element having different masses, due to varying numbers of neutrons. �Ex) Carbon-14, Uranium-235

Organize the Candy! � Each table will be given a cup of 11 different candies. Consider these candies to be different “elements” � Create a table with multiple rows and columns with the candy. There should be patterns/organization within the table *When organizing, consider what makes up the candy? What size is it? What company? etc. *Which row will have chocolate? Which row will have yellow candy? , etc. � After 5 minutes, groups will then share and explain how they arranged their table

Questions to consider � How did you arrange your rows? � How did you arrange your columns? � Are there any candies that would fit well to your trends? � What things did you consider when arranging your candies?

Periodic Table
Questions �How �Why is the modern Periodic Table organized? do the elements in a group have similar properties?
Periodic Table - History Dmitri Mendeleev: ● "Father of the Periodic Table" ● Mid-1800's Organized table by elements’ chemical & physical properties ● Open spots in table = undiscovered elements ● o Correctly predicted their properties’s o Originally only 63 elements, now there 118
Periodic Table - History Mendeleev came up with the “Periodic Law of the Elements. ” Periodic Law – properties of elements change in a relationship to atomic number that repeats. ● "Periodic" = repeating pattern

Periodic Table - History Henry Mosley: ● Organized table by periods and groups. The original Periodic Table was organized horizontally by increasing mass & vertically by similar properties.
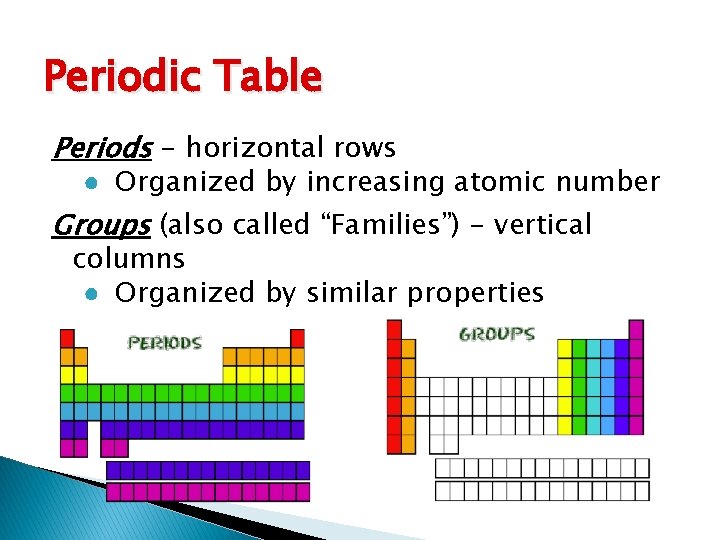
Periodic Table Periods - horizontal rows ● Organized by increasing atomic number Groups (also called “Families”) - vertical columns ● Organized by similar properties

Periodic Table Directions: 1. As you read through the presentation, color the groups of your periodic table accordingly using colored pencils. 2. Include a key that details the names of each group you colored.

Group 1 - Alkali Metals � Group: 1 � Properties: Soft & very reactive � # of Valence Electrons: 1 Color: dark green

Group 2 - Alkaline Earth Metals � Group: 2 � Properties: React with acids to produce hydrogen � # of Valence Electrons: 2 Color: light green

Group 17 - Halogens � Group: 17 � Properties: React with metals to form salts � # of Valence Electrons: 7 Color: yellow
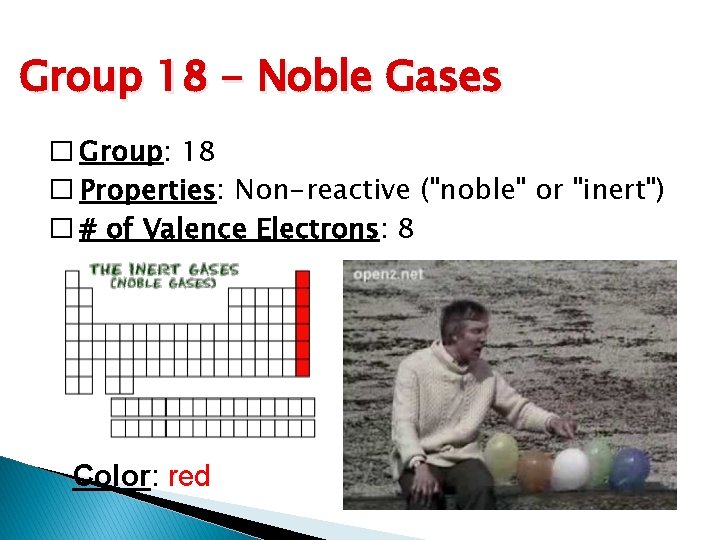
Group 18 - Noble Gases � Group: 18 � Properties: Non-reactive ("noble" or "inert") � # of Valence Electrons: 8 Color: red

Video: Noble Gases Helium is a noble gas that is six times less dense than air. Xenon is a noble gas that is six times more dense than air.
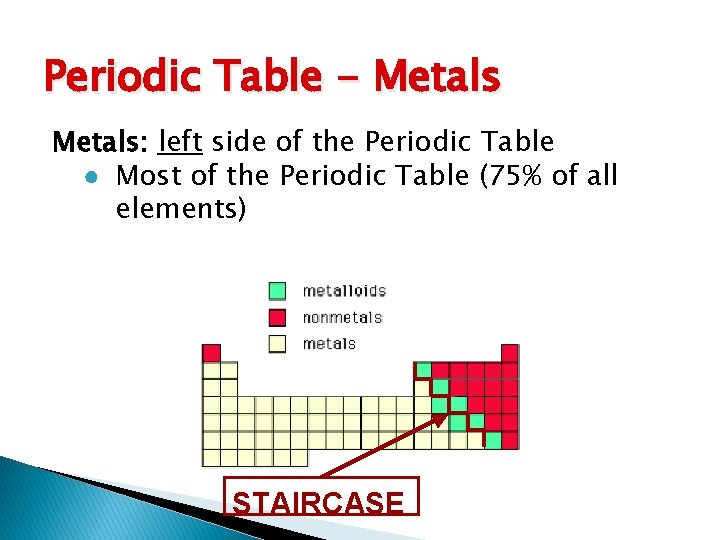
Periodic Table - Metals: left side of the Periodic Table ● Most of the Periodic Table (75% of all elements) STAIRCASE
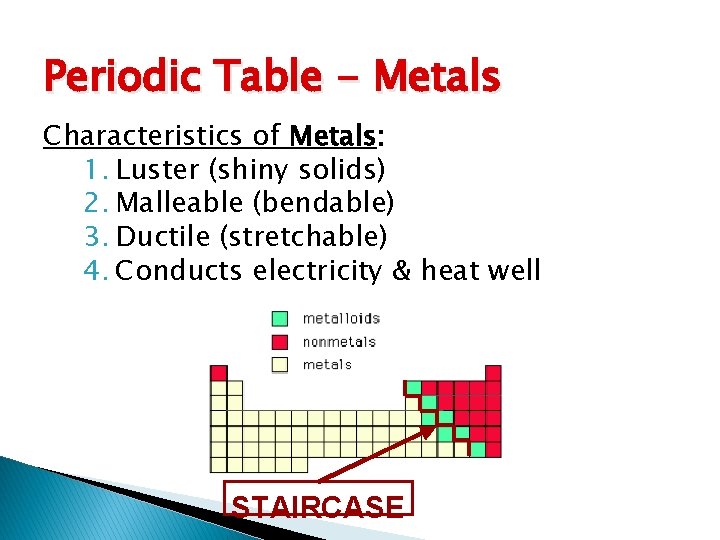
Periodic Table - Metals Characteristics of Metals: 1. Luster (shiny solids) 2. Malleable (bendable) 3. Ductile (stretchable) 4. Conducts electricity & heat well STAIRCASE
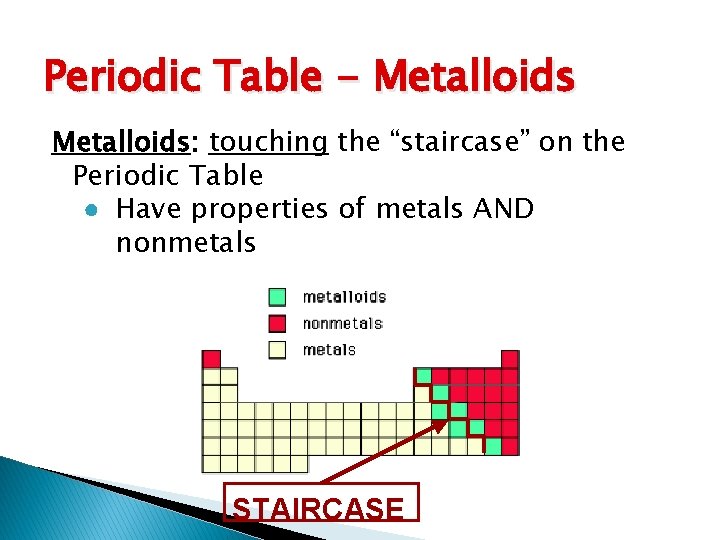
Periodic Table - Metalloids: touching the “staircase” on the Periodic Table ● Have properties of metals AND nonmetals STAIRCASE
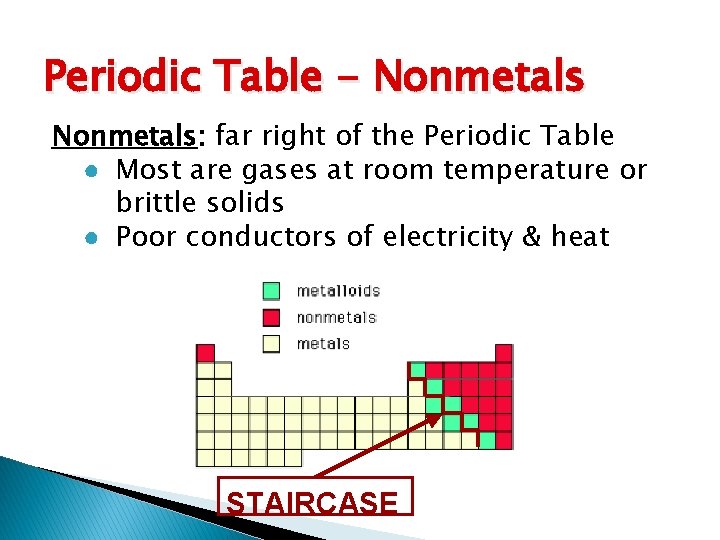
Periodic Table - Nonmetals: far right of the Periodic Table ● Most are gases at room temperature or brittle solids ● Poor conductors of electricity & heat STAIRCASE
Periodic Table - Summary
- Slides: 25