Atoms Elements and Compounds Chapter Eight Molecules and












- Slides: 19


Atoms, Elements, and Compounds

Chapter Eight: Molecules and Compounds • Compounds and Chemical Bonds • Electrons and Chemical Bonds

Investigation 15 B Molecules and Compounds • What are some molecules and compounds and what atoms are in them?

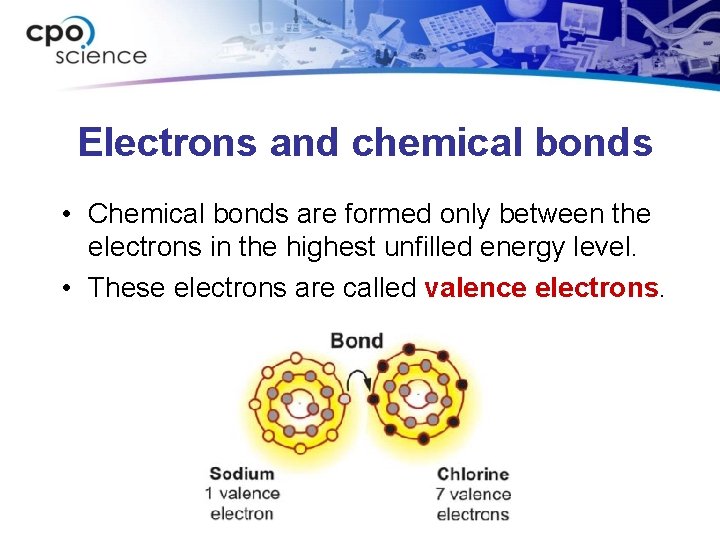
Electrons and chemical bonds • Chemical bonds are formed only between the electrons in the highest unfilled energy level. • These electrons are called valence electrons.


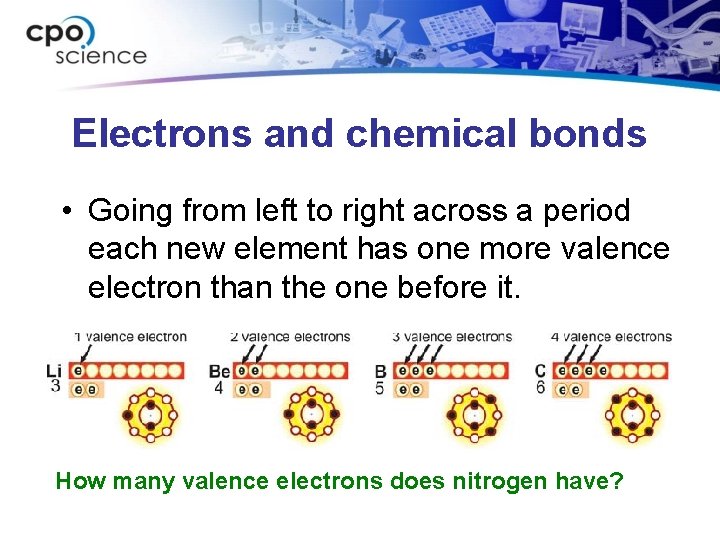
Electrons and chemical bonds • Going from left to right across a period each new element has one more valence electron than the one before it. How many valence electrons does nitrogen have?

Lewis dot diagrams • A clever way to keep track of valence electrons is to draw Lewis dot diagrams. • A dot diagram shows the element symbol surrounded by one to eight dots representing What is the dot the valence electrons. structure for nitrogen?


Oxidation numbers • An oxidation number indicates the charge on the remaining atom (ion) when electrons are lost, gained, or shared in chemical bonds. • A sodium atom always ionizes to become Na+ (a charge of +1) when it combines with other atoms to make a compound. • Therefore, we say that sodium has an oxidation number of 1+. What is the most common oxidation number for nitrogen?


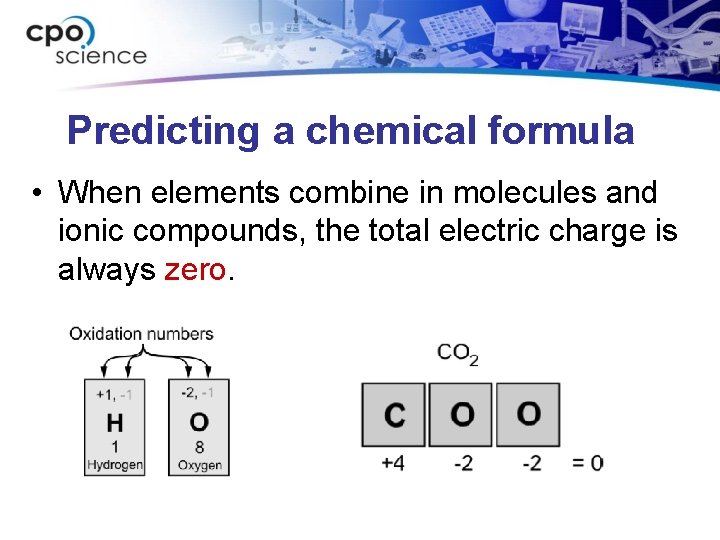
Predicting a chemical formula • When elements combine in molecules and ionic compounds, the total electric charge is always zero.



Ionic and covalent bonds • On the periodic table, strong electron donors are the left side (alkali metals). • Strong electron acceptors are on the right side (halogens). • The further apart two elements are on the periodic table, the more likely they are to form an ionic compound.


Ionic and covalent bonds • Covalent compounds form when elements have roughly equal tendency to accept electrons. • Elements that are both nonmetals and therefore close together on the periodic table tend to form covalent compounds.

Technology Connection Spiderman’s Favorite Compound • The physical properties of gold make it useful in surprising ways. • You can find gold in astronaut gear, airplane windshields, and even in some people’s eyelids!

Activity Plastics and Evaporation • Plastic wrap has many household uses. • In this activity, you will investigate different polymers used in plastic wrap.