Atoms Development of the Atomic Theory 1 Democritus




- Slides: 20

Atoms: Development of the Atomic Theory 1

Democritus n 460 BC - Greek philosopher proposes the existence of the atom n He pounded materials until he made them into smaller and smaller parts n He called them “atoma” which is Greek for “indivisible”.

Democritus n. His Theory: All atoms: n Are small hard particles n Are made of a single material formed into different shapes and sizes n Are always moving, and they form different materials by joining together

John Dalton 1803 - British chemist n Elements combine in specific proportions to form compounds n Solid Sphere Model or Billiard Ball Model Proposed by John Dalton

John Dalton n His Theory: § All substances are made of atoms that cannot be created, divided, or destroyed. § Atoms join with other atoms to make new substances. § Atoms of the same element are exactly alike, and atoms of different elements are different in mass and size.

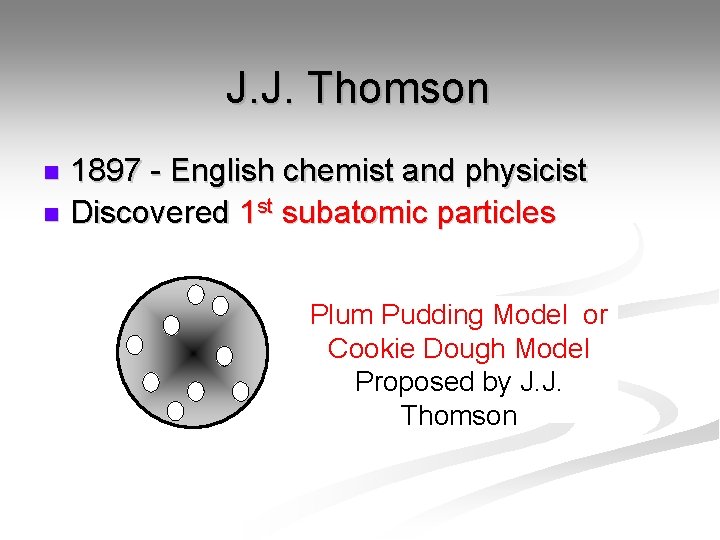
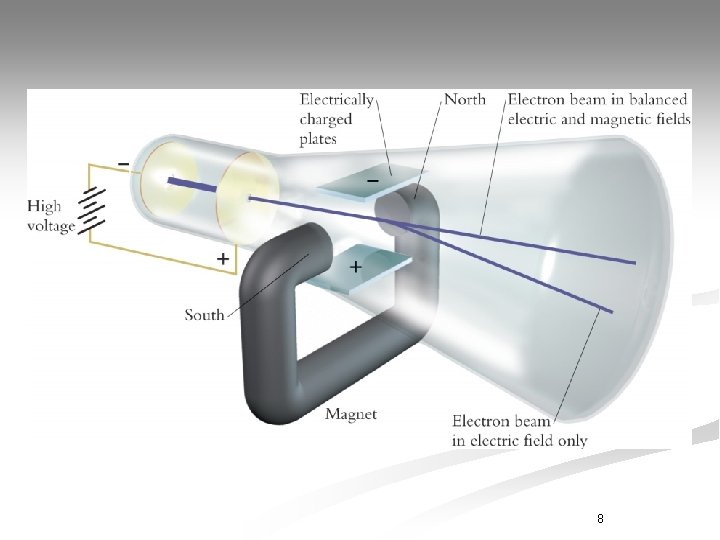
J. J. Thomson 1897 - English chemist and physicist n Discovered 1 st subatomic particles n Plum Pudding Model or Cookie Dough Model Proposed by J. J. Thomson

J. J. Thomson n. His Theory: n Used Cathode Ray Tube to conduct experiments. n Atoms contain negatively charged particles called electrons and positively charged matter. n Created a model to describe the atom as a sphere filled with positive matter with negative particles mixed in

8

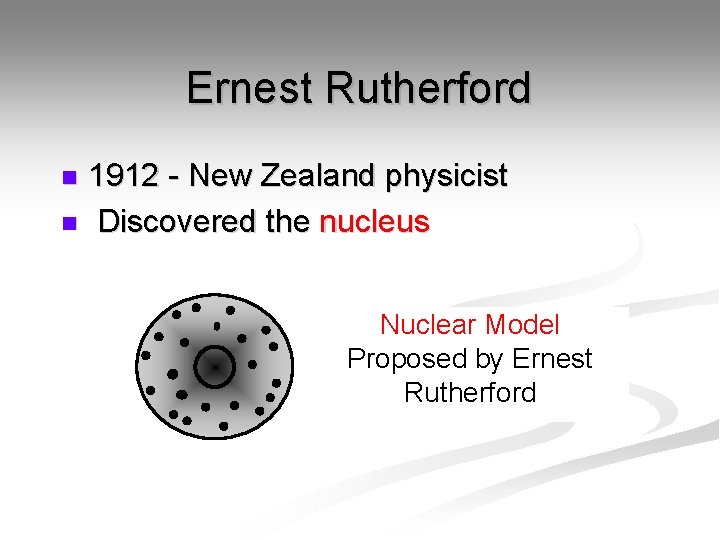
Ernest Rutherford 1912 - New Zealand physicist n Discovered the nucleus n Nuclear Model Proposed by Ernest Rutherford

10

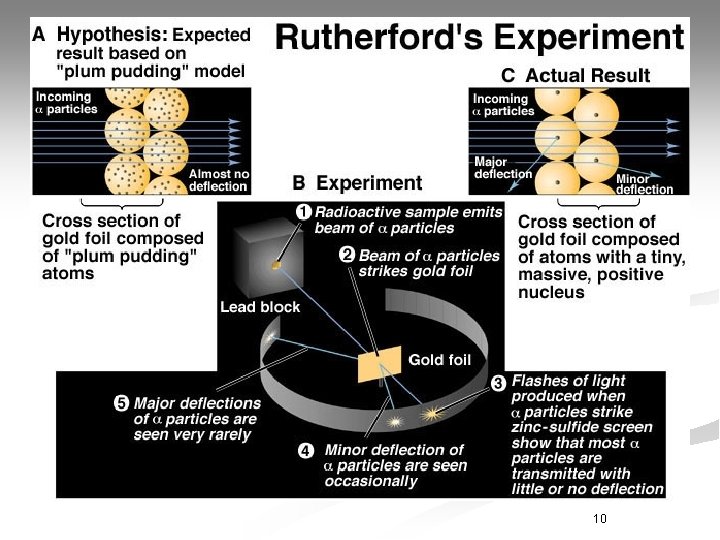
Ernest Rutherford n. His Theory: § Used Gold Foil Experiment for his research. § Small, dense, positively charged particle present in nucleus called a proton § Electrons travel around the nucleus, but their exact places cannot be described.

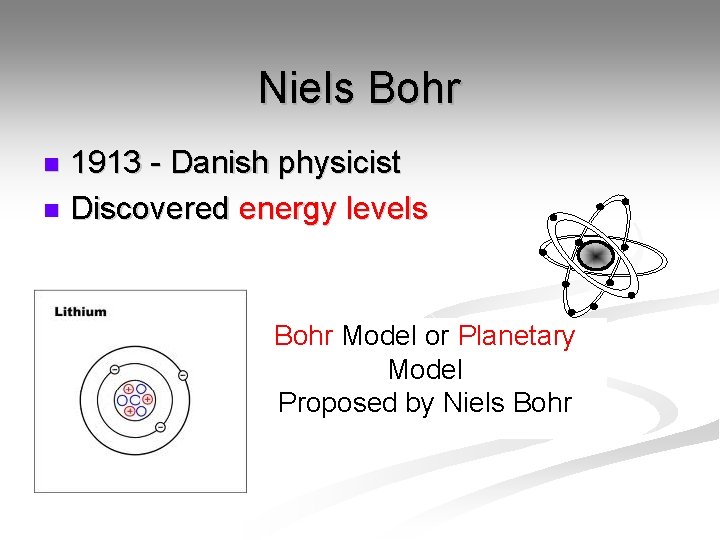
Niels Bohr 1913 - Danish physicist n Discovered energy levels n Bohr Model or Planetary Model Proposed by Niels Bohr

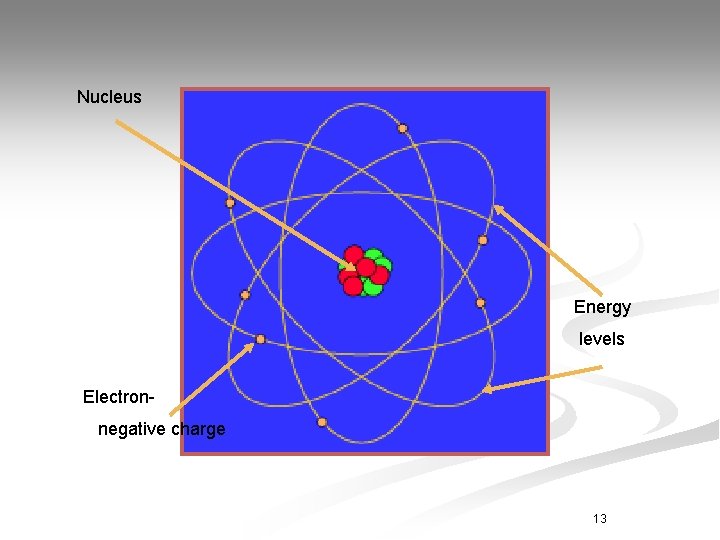
Nucleus Energy levels Electronnegative charge 13

Niels Bohr n. His Theory: § Electrons travel around the nucleus in definite paths and fixed distances. § Electrons can jump from one level to a path in another level.

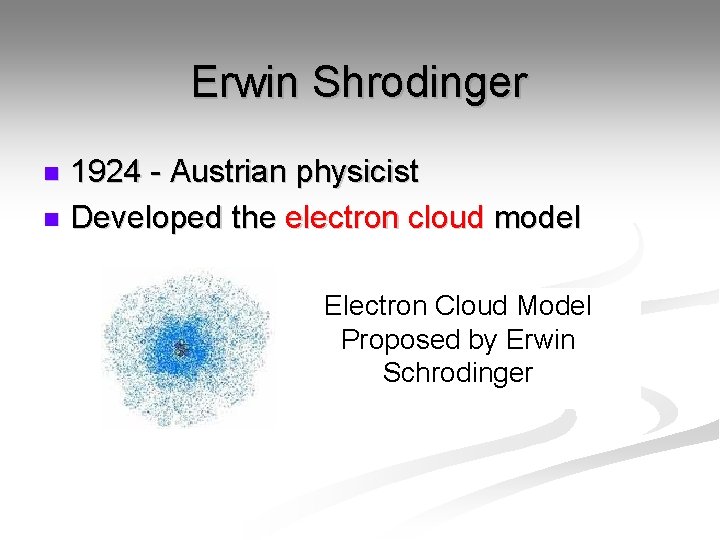
Erwin Shrodinger 1924 - Austrian physicist n Developed the electron cloud model n Electron Cloud Model Proposed by Erwin Schrodinger

Erwin Shrodinger n. His Theory: § The exact path of electrons cannot be predicted. § The region referred to as the electron cloud, is an area where electrons can likely be found.

Modern Theory of the Atom n Atoms are composed of three main subatomic particles: the electron, proton, and neutron. n Most of the mass of the atom is concentrated in the nucleus of the atom.

Modern Theory of the Atom n The protons and neutrons are located within the nucleus, while the electrons exist outside of the nucleus. n In neutral atoms, the number of protons is equal to the number of electrons.

Modern Theory of the Atom n The type of atom is determined by the number of protons it has. n The number of protons in an atom is equal to the atomic number.

Modern Theory of the Atom n The sum of the number of protons and neutrons in a particular atom is called the mass number. n Valence electrons are the outermost electrons.