Atoms Definition the smallest particles of an element


- Slides: 11

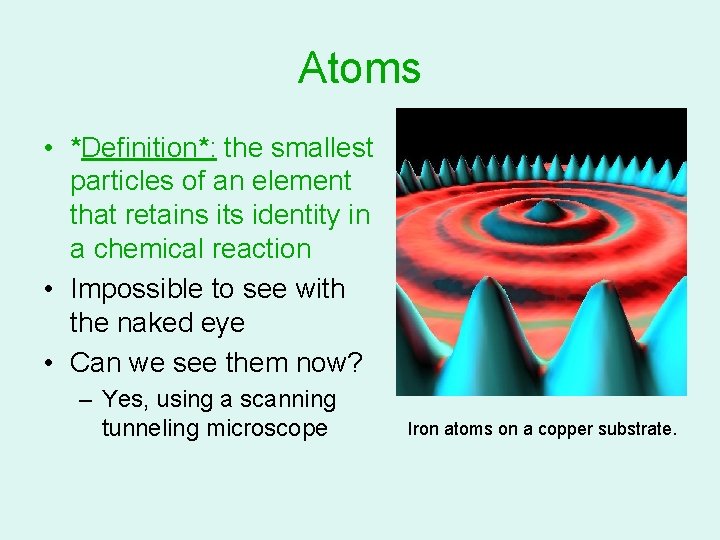
Atoms • *Definition*: the smallest particles of an element that retains its identity in a chemical reaction • Impossible to see with the naked eye • Can we see them now? – Yes, using a scanning tunneling microscope Iron atoms on a copper substrate.

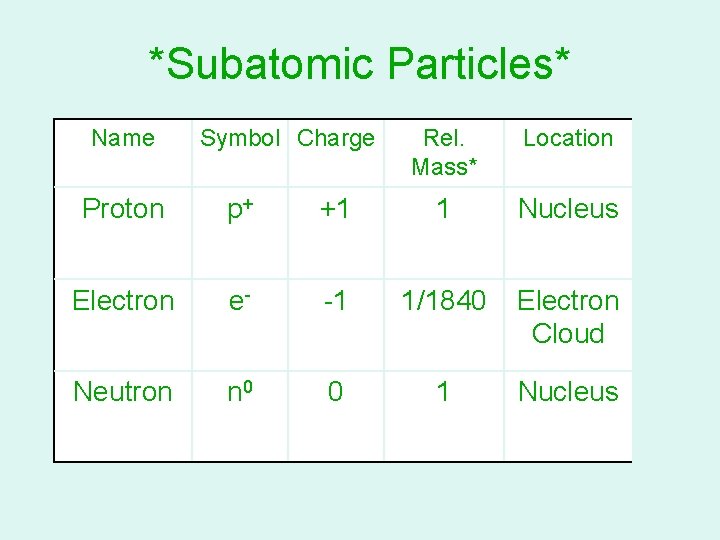
NCSCOS 2. 02 *Subatomic Particles* Name Symbol Charge Rel. Mass* Location Proton p+ +1 1 Nucleus Electron e- -1 1/1840 Electron Cloud Neutron n 0 0 1 Nucleus *Actual masses can be found on your yellow sheets

If all atoms are made up of protons, neutrons, and electrons; what makes elements different from each other? Answer: They have different numbers of protons

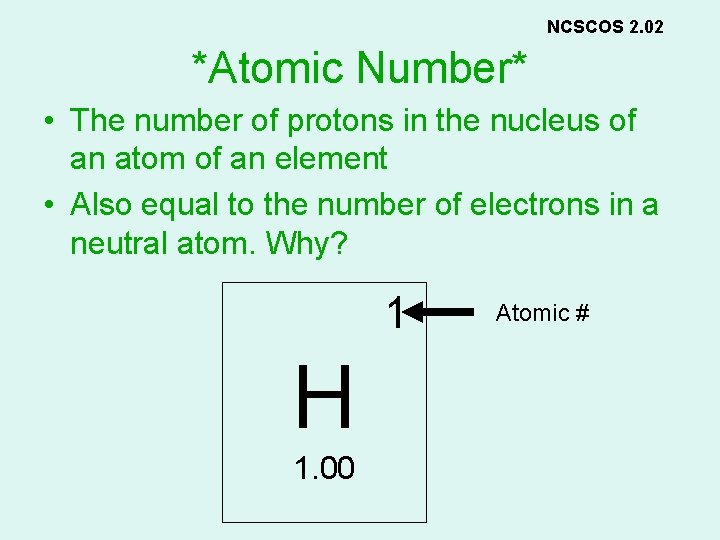
NCSCOS 2. 02 *Atomic Number* • The number of protons in the nucleus of an atom of an element • Also equal to the number of electrons in a neutral atom. Why? 1 H 1. 00 Atomic #

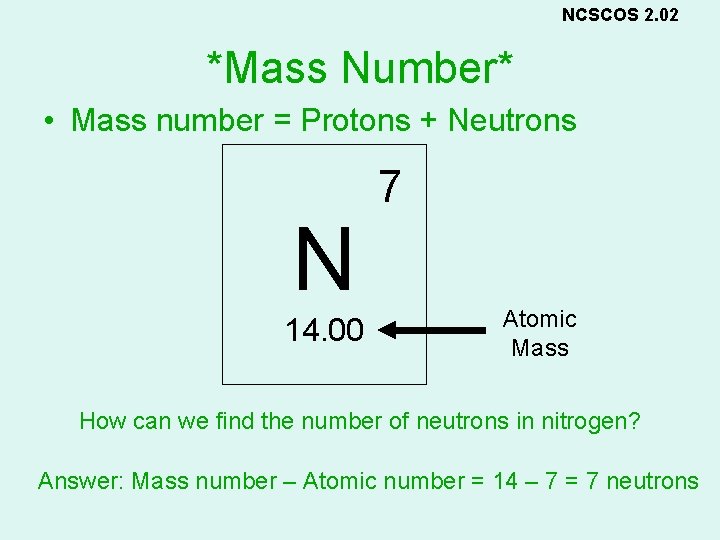
NCSCOS 2. 02 *Mass Number* • Mass number = Protons + Neutrons N 14. 00 7 Atomic Mass How can we find the number of neutrons in nitrogen? Answer: Mass number – Atomic number = 14 – 7 = 7 neutrons

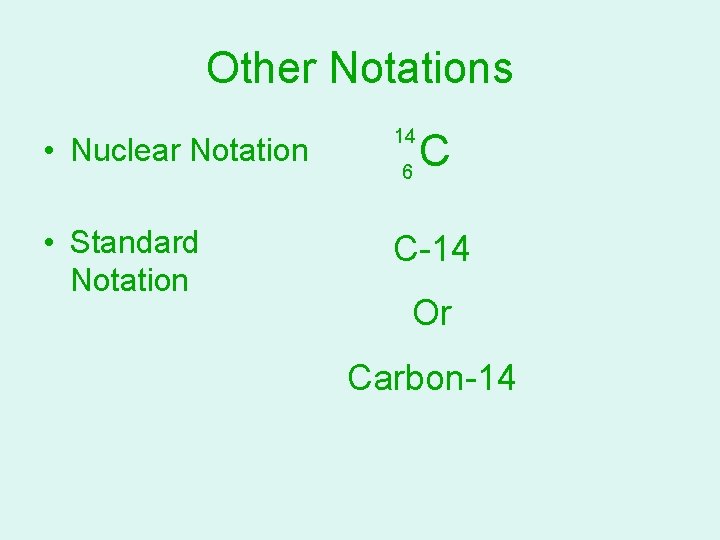
Other Notations • Nuclear Notation 14 • Standard Notation C-14 6 C Or Carbon-14

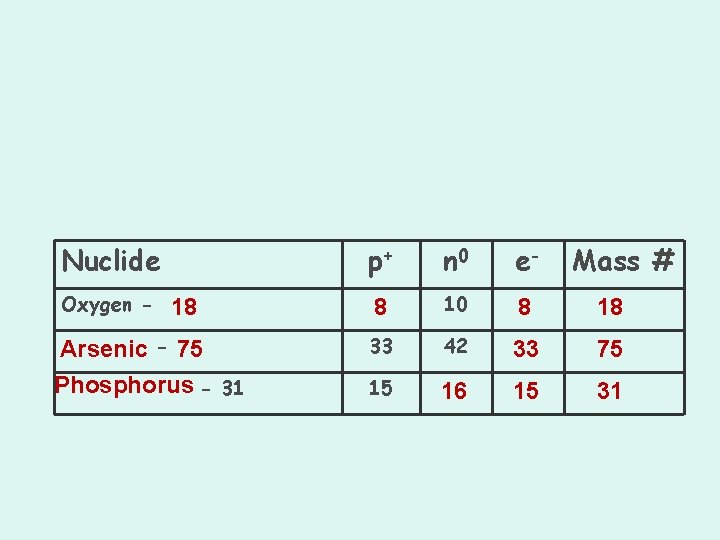
Nuclide Oxygen - 18 Arsenic - 75 Phosphorus - 31 p+ n 0 e- Mass # 8 10 8 18 33 42 33 75 15 16 15 31

• Why aren’t all mass numbers whole numbers? • *Isotopes* – An isotope of an element has the same number of protons but different numbers of neutrons – Ex: Neon has 3 isotopes • Neon-20 • Neon-21 • Neon-22 • The mass number on the periodic table is a weighted average of all of the isotopes of the element.

Weighted Average • Multiply the numbers by their percentages in decimal form, then add together. • Ex: Grades – Our grading system is a percentage based system • • Tests 40% Quizzes 15% Labs 25% Homework 20%

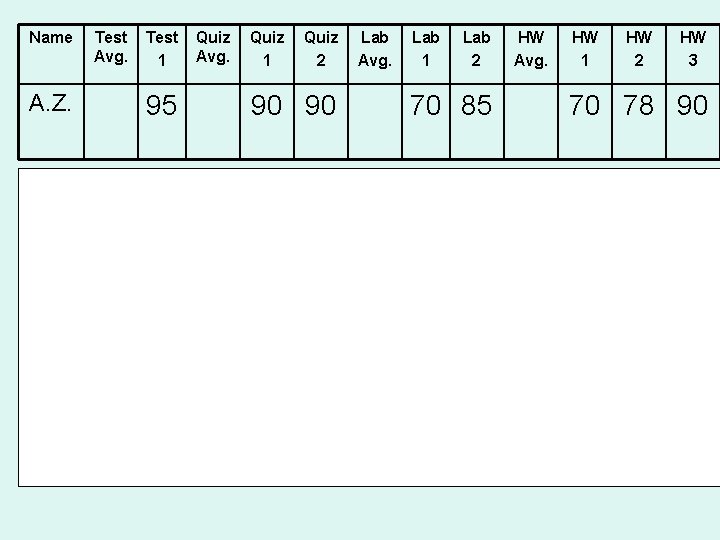
Name A. Z. Test Avg. Test 1 95 Quiz Avg. Quiz 1 Quiz 2 90 90 Lab Avg. Lab 1 Lab 2 70 85 HW Avg. HW 1 HW 2 HW 3 70 78 90 Average out each set of grades first: Quiz: 90+90=180/2=90 Now multiply these averages by their percentages (35 x 85) + (25 x 80) + (20 x 76) = 8095 Your percentages should be expressed as decimals they are multiplied by the averages Sanity Check! before Does this make since? (. 35 x 85) + (. 25 x 80. ) + (. 20 x 76) = 81

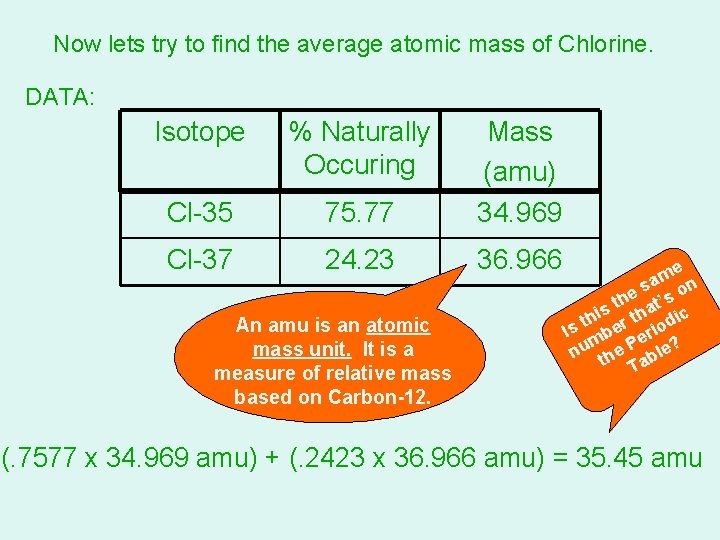
Now lets try to find the average atomic mass of Chlorine. DATA: Isotope % Naturally Occuring Cl-35 75. 77 Mass (amu) 34. 969 Cl-37 24. 23 36. 966 An amu is an atomic mass unit. It is a measure of relative mass based on Carbon-12. e m sa on e th at’s s i th er th iodic s I b er m nu the P ble? Ta (. 7577 x 34. 969 amu) + (. 2423 x 36. 966 amu) = 35. 45 amu