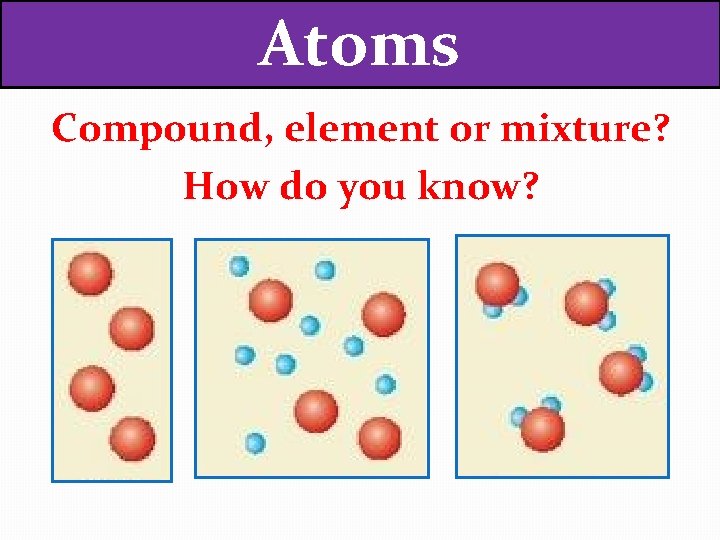
Atoms Compound element or mixture How do you





























- Slides: 74

Atoms Compound, element or mixture? How do you know?

Homework Due in: Wed 8 th Nov TASK Complete the SMH quiz 3 times!

Learning Objectives TWWL – about compounds, elements and mixtures – State some key ideas about atoms – Describe how atoms are different to each other – Explain how atoms are positioned in the periodic table

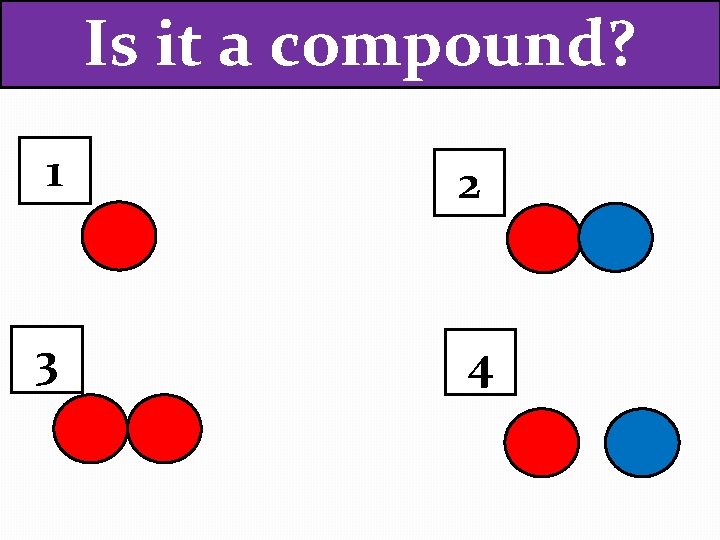
Is it a compound? 1 2 3 4

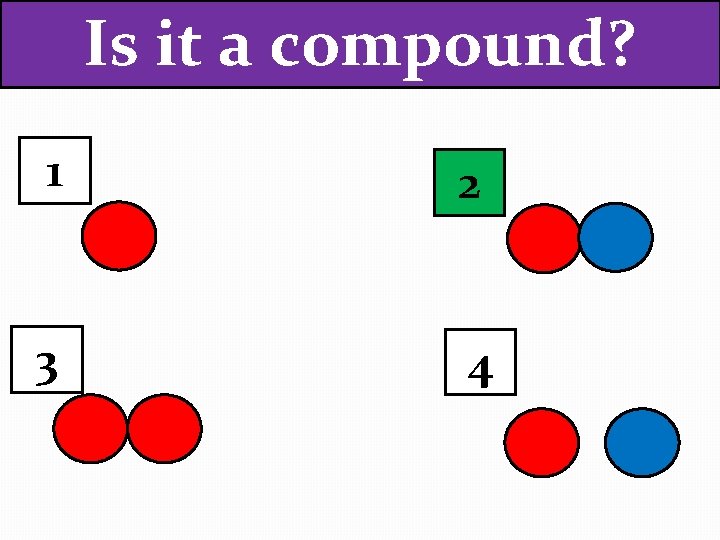
Is it a compound? 1 2 3 4

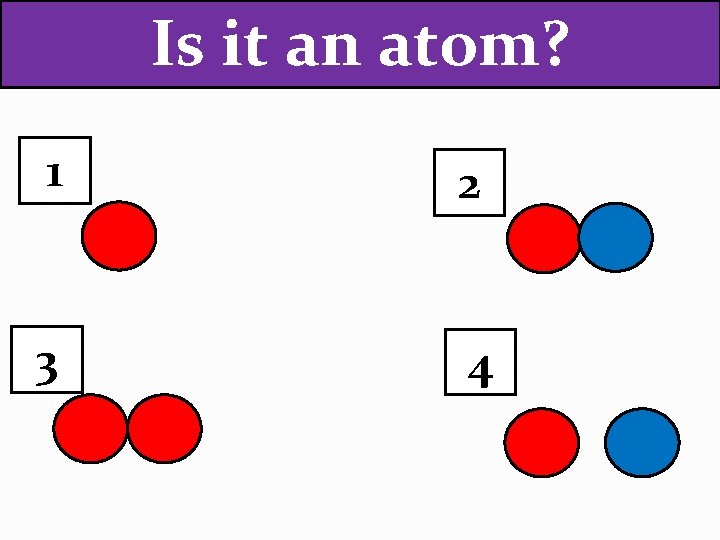
Is it an atom? 1 2 3 4

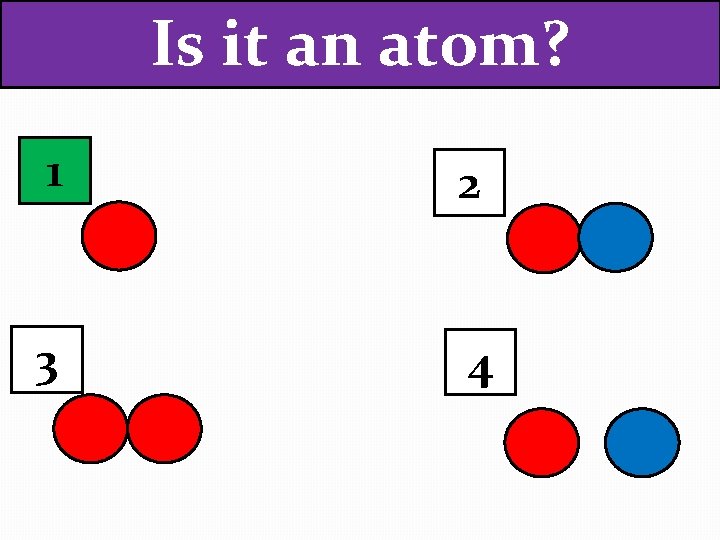
Is it an atom? 1 2 3 4

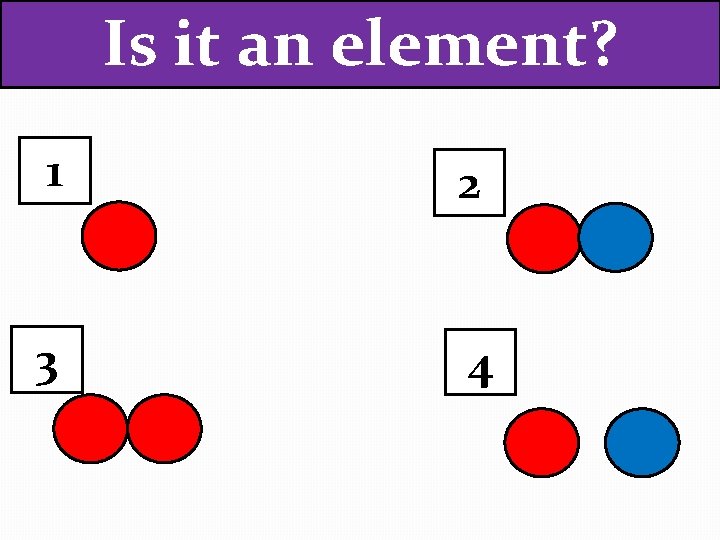
Is it an element? 1 2 3 4

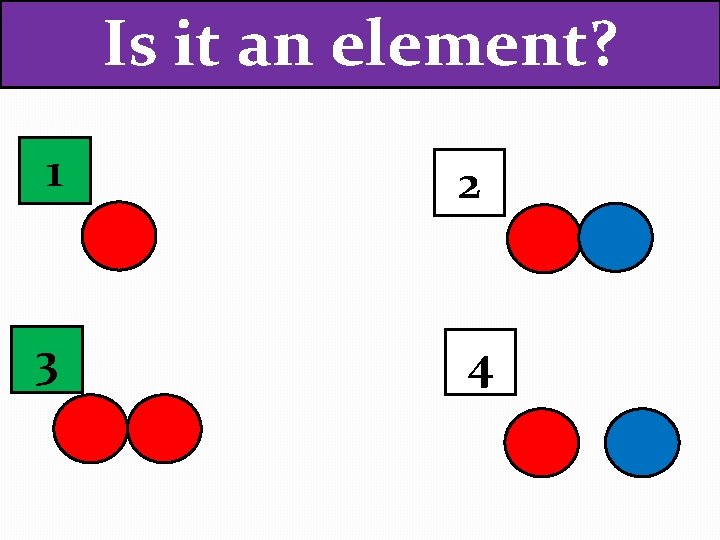
Is it an element? 1 2 3 4

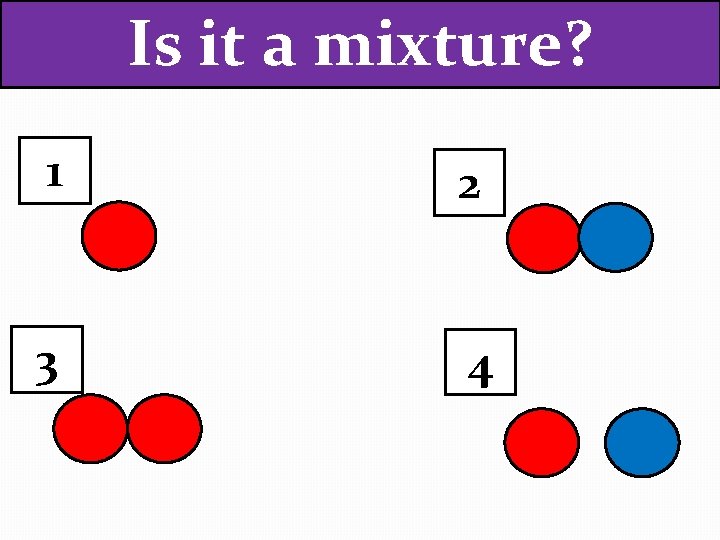
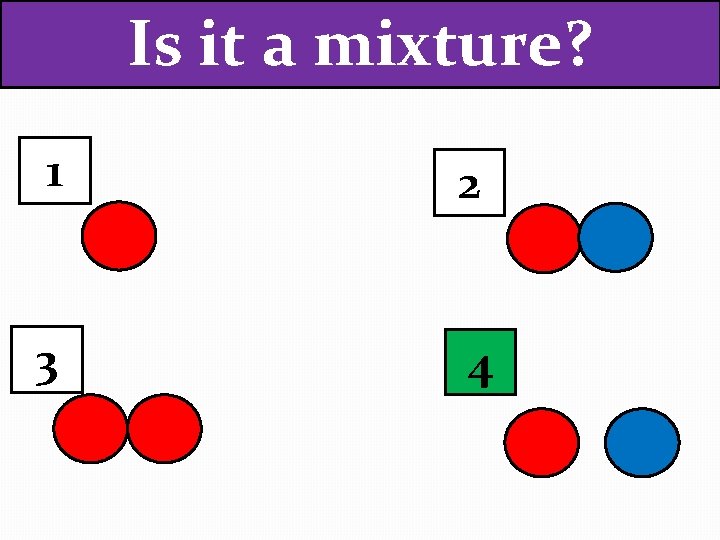
Is it a mixture? 1 2 3 4

Is it a mixture? 1 2 3 4

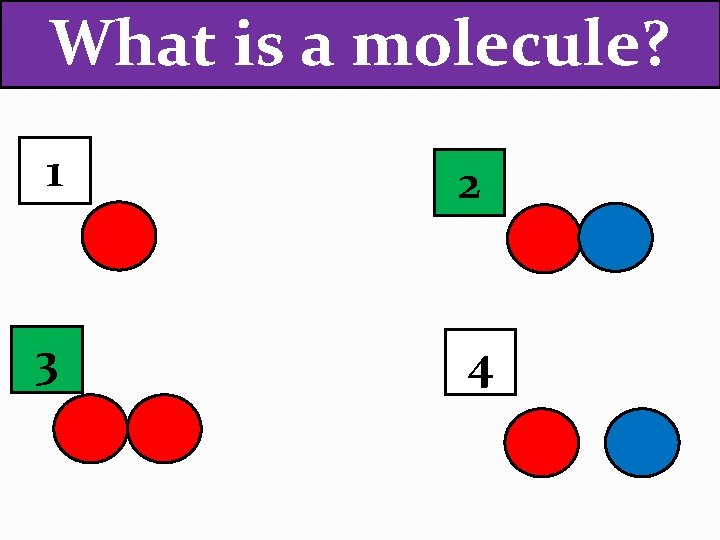
What is a molecule? 1 2 3 4

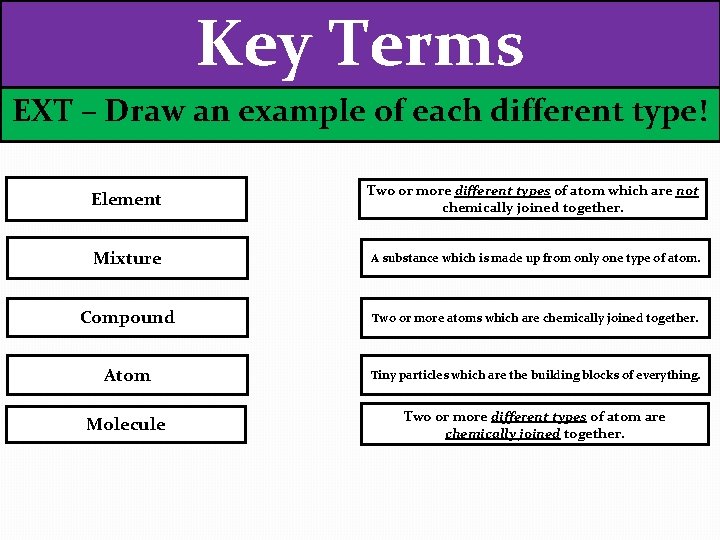
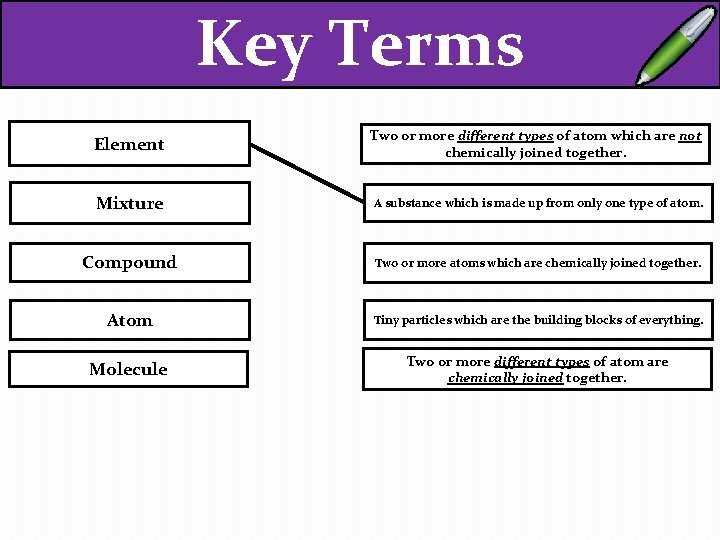
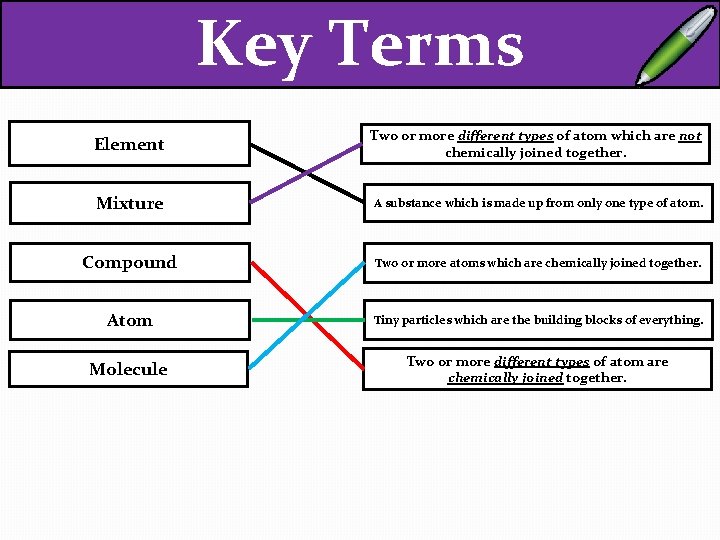
Key Terms EXT – Draw an example of each different type! Element Two or more different types of atom which are not chemically joined together. Mixture A substance which is made up from only one type of atom. Compound Two or more atoms which are chemically joined together. Atom Tiny particles which are the building blocks of everything. Molecule Two or more different types of atom are chemically joined together.

Key Terms Element Two or more different types of atom which are not chemically joined together. Mixture A substance which is made up from only one type of atom. Compound Two or more atoms which are chemically joined together. Atom Tiny particles which are the building blocks of everything. Molecule Two or more different types of atom are chemically joined together.

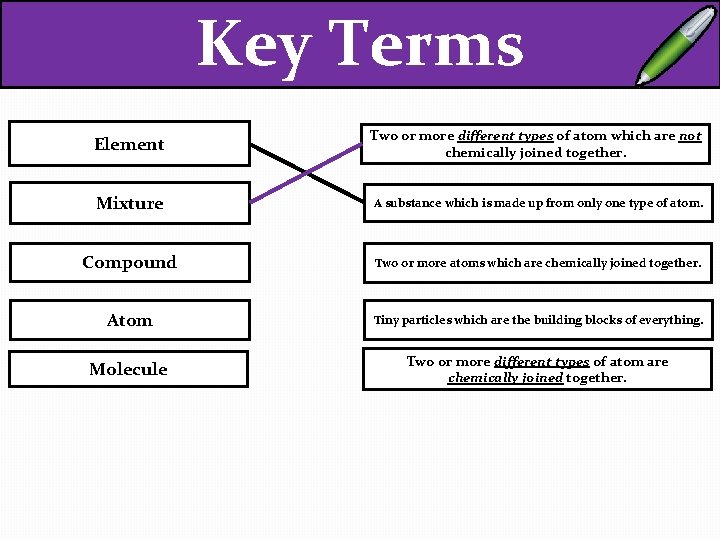
Key Terms Element Two or more different types of atom which are not chemically joined together. Mixture A substance which is made up from only one type of atom. Compound Two or more atoms which are chemically joined together. Atom Tiny particles which are the building blocks of everything. Molecule Two or more different types of atom are chemically joined together.

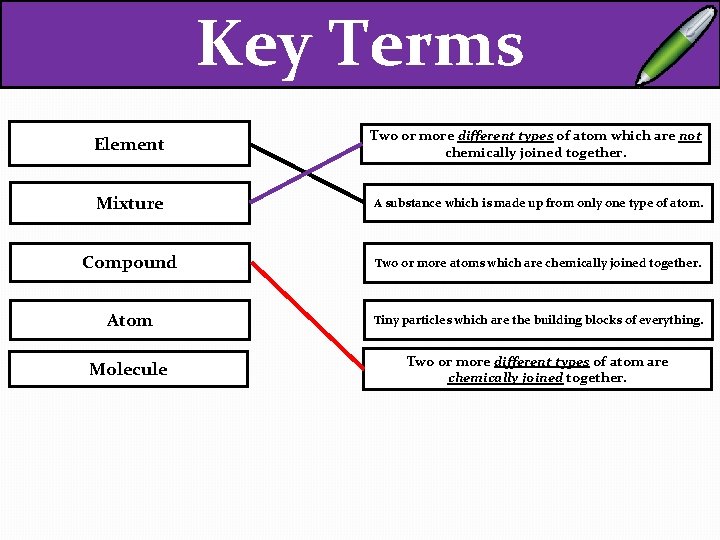
Key Terms Element Two or more different types of atom which are not chemically joined together. Mixture A substance which is made up from only one type of atom. Compound Two or more atoms which are chemically joined together. Atom Tiny particles which are the building blocks of everything. Molecule Two or more different types of atom are chemically joined together.

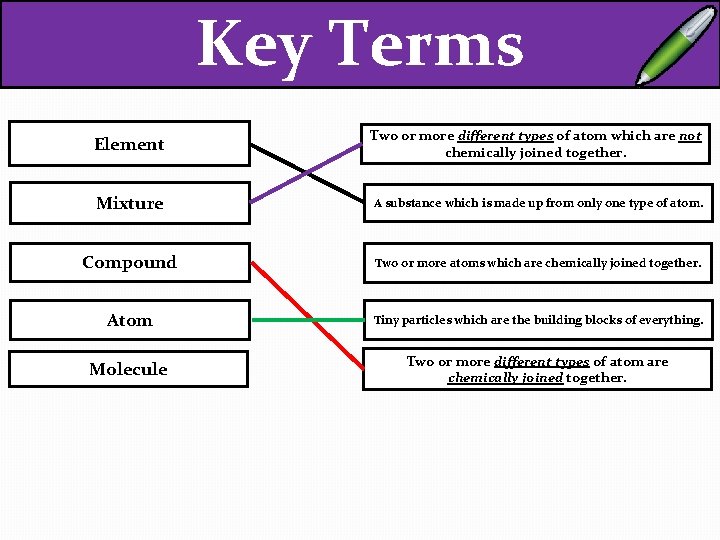
Key Terms Element Two or more different types of atom which are not chemically joined together. Mixture A substance which is made up from only one type of atom. Compound Two or more atoms which are chemically joined together. Atom Tiny particles which are the building blocks of everything. Molecule Two or more different types of atom are chemically joined together.

Key Terms Element Two or more different types of atom which are not chemically joined together. Mixture A substance which is made up from only one type of atom. Compound Two or more atoms which are chemically joined together. Atom Tiny particles which are the building blocks of everything. Molecule Two or more different types of atom are chemically joined together.

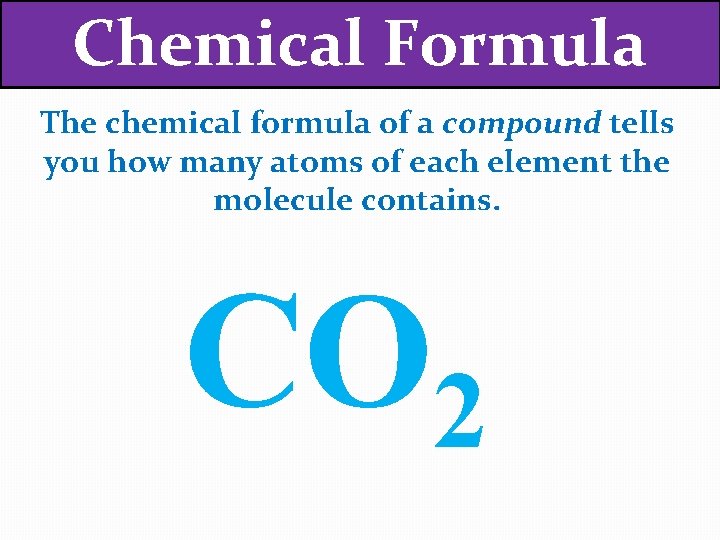
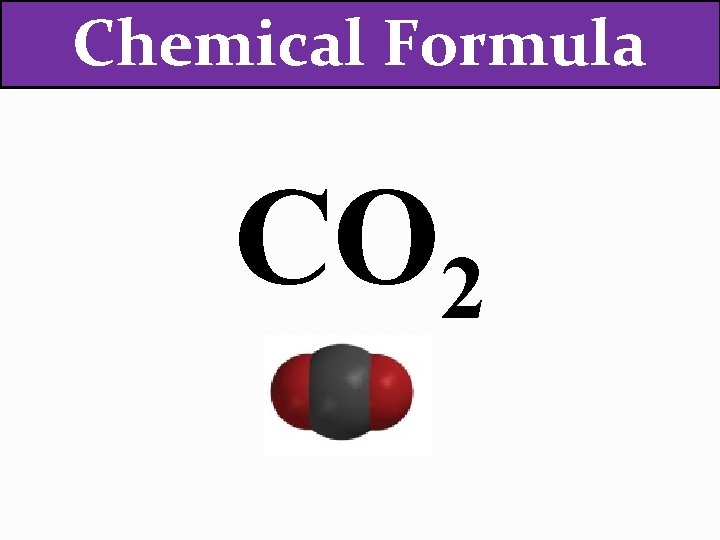
Chemical Formula The chemical formula of a compound tells you how many atoms of each element the molecule contains. CO 2

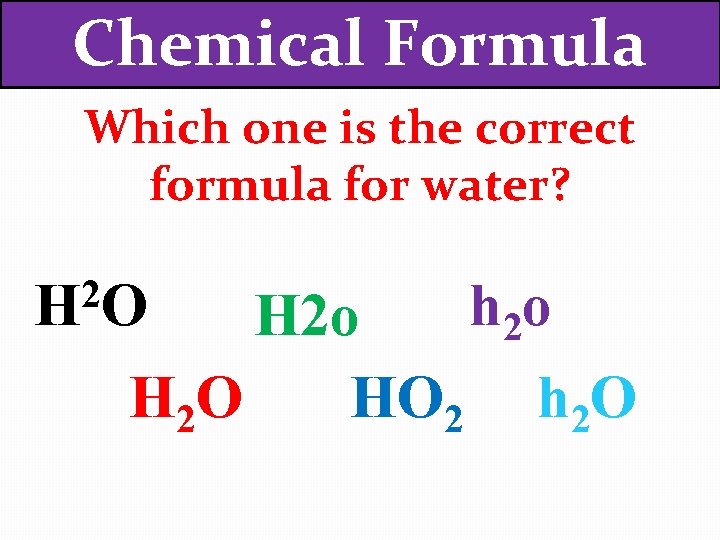
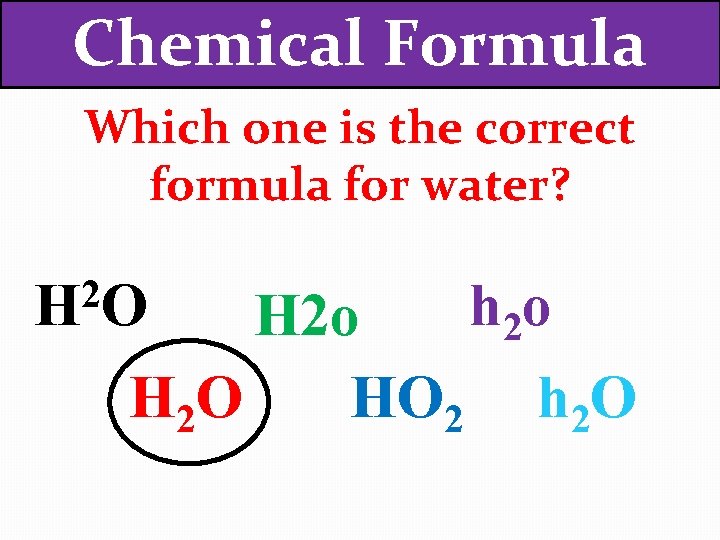
Chemical Formula Which one is the correct formula for water? 2 HO h 2 o HO 2 h 2 O H 2 O

Chemical Formula Which one is the correct formula for water? 2 HO h 2 o HO 2 h 2 O H 2 O

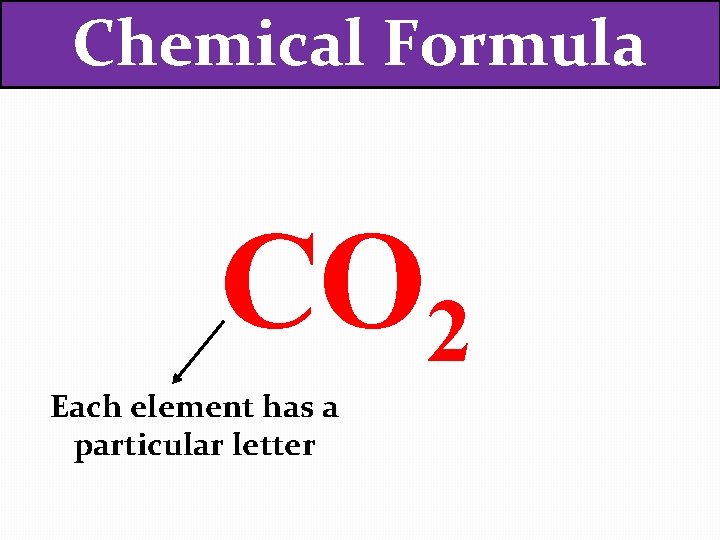
Chemical Formula CO 2 Each element has a particular letter

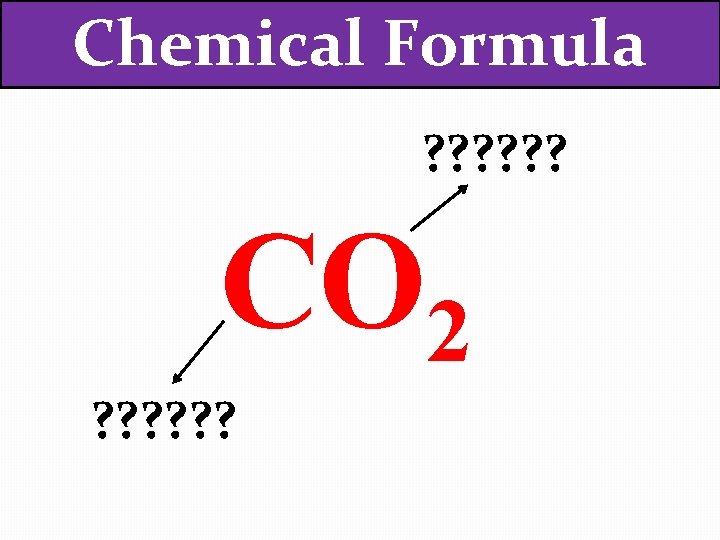
Chemical Formula ? ? ? CO 2 ? ? ?

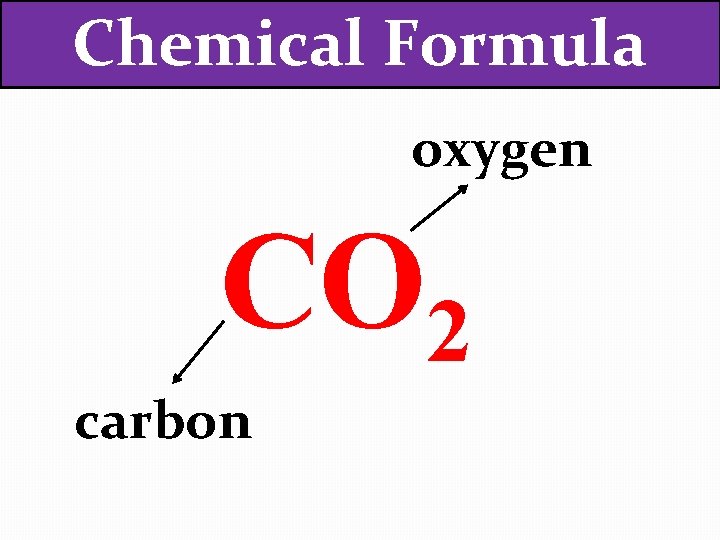
Chemical Formula oxygen CO 2 carbon

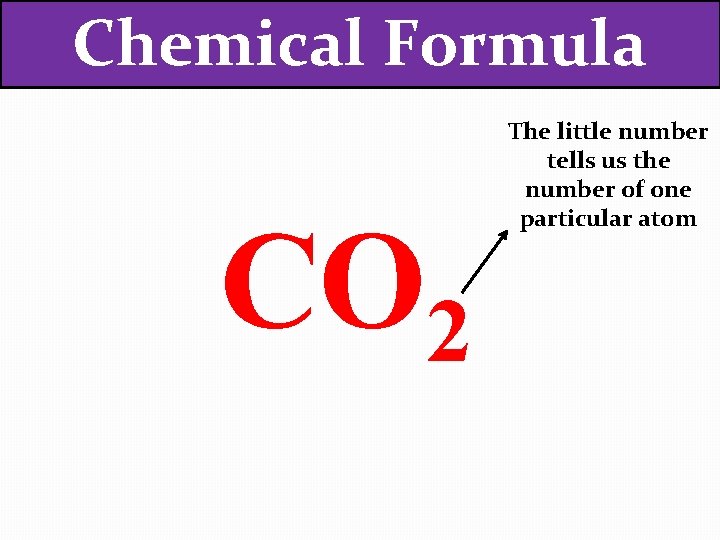
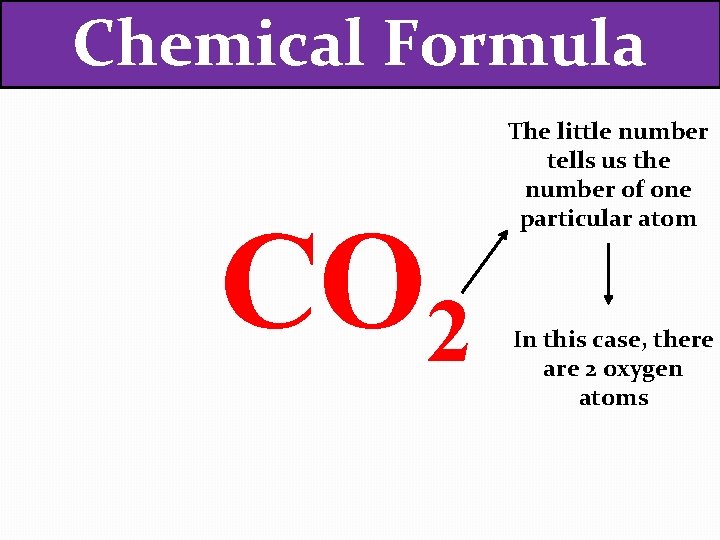
Chemical Formula CO 2 The little number tells us the number of one particular atom

Chemical Formula CO 2 The little number tells us the number of one particular atom In this case, there are 2 oxygen atoms

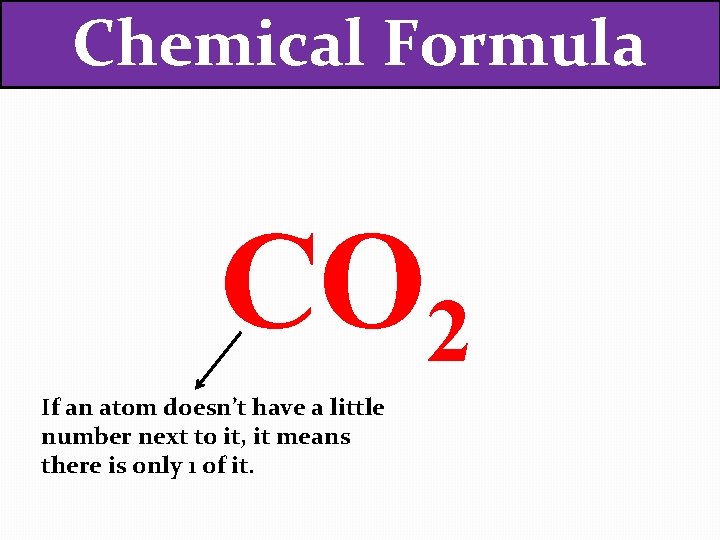
Chemical Formula CO 2 If an atom doesn’t have a little number next to it, it means there is only 1 of it.

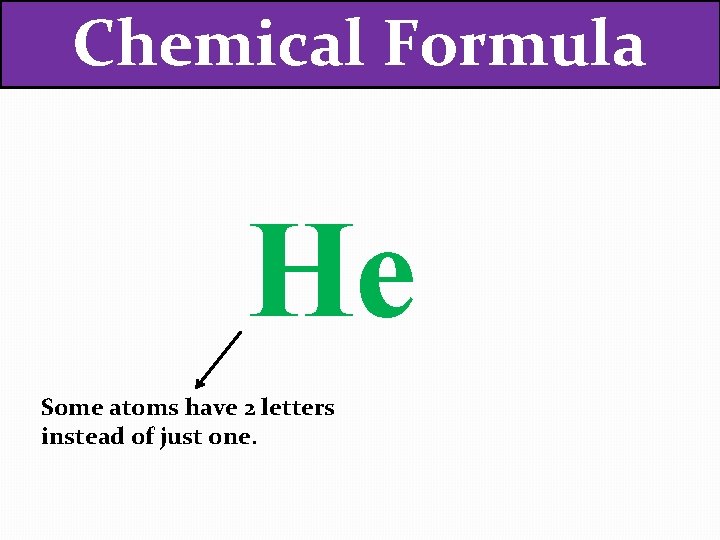
Chemical Formula He Some atoms have 2 letters instead of just one.

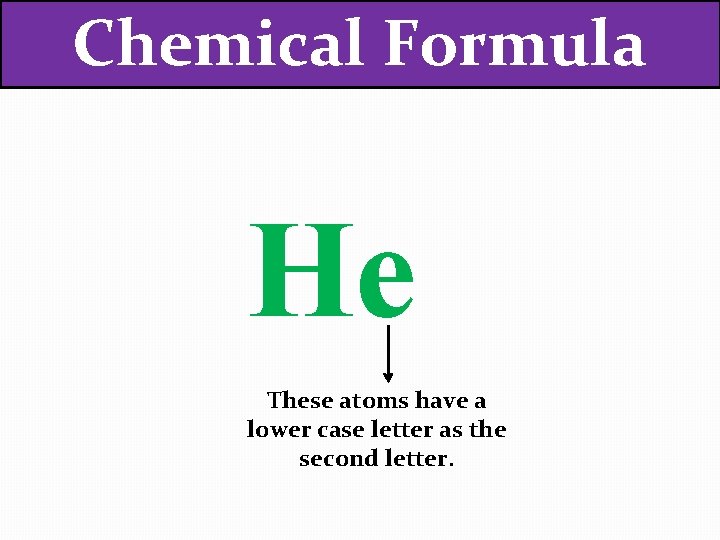
Chemical Formula He These atoms have a lower case letter as the second letter.

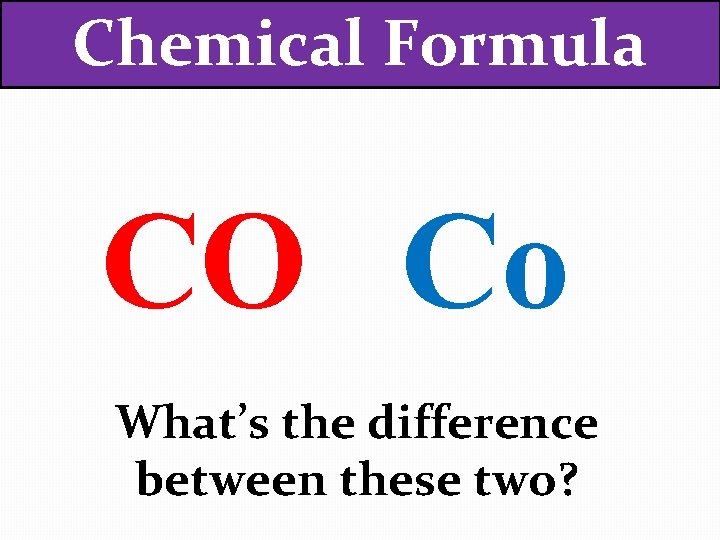
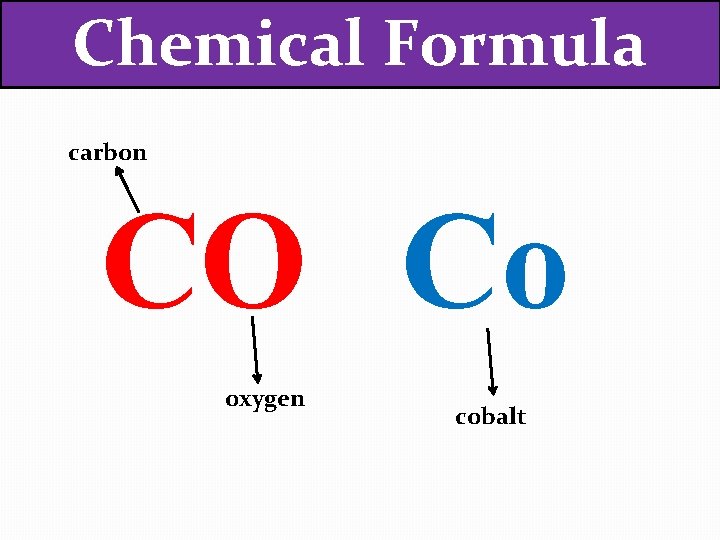
Chemical Formula CO Co What’s the difference between these two?

Chemical Formula carbon CO Co oxygen cobalt

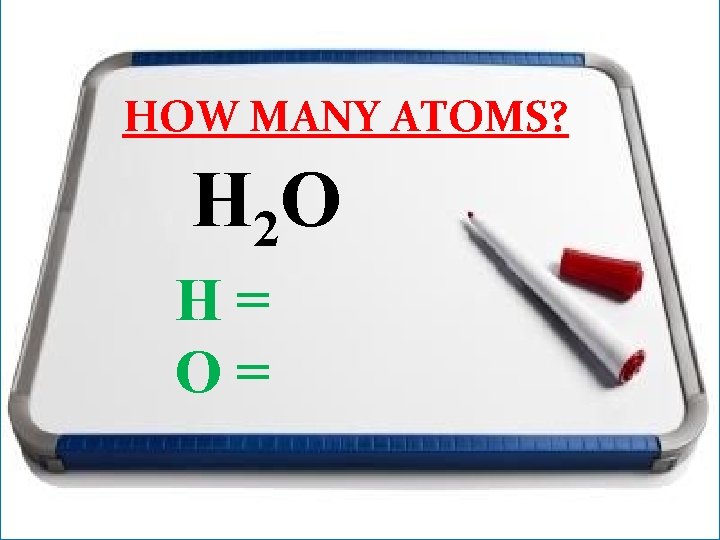
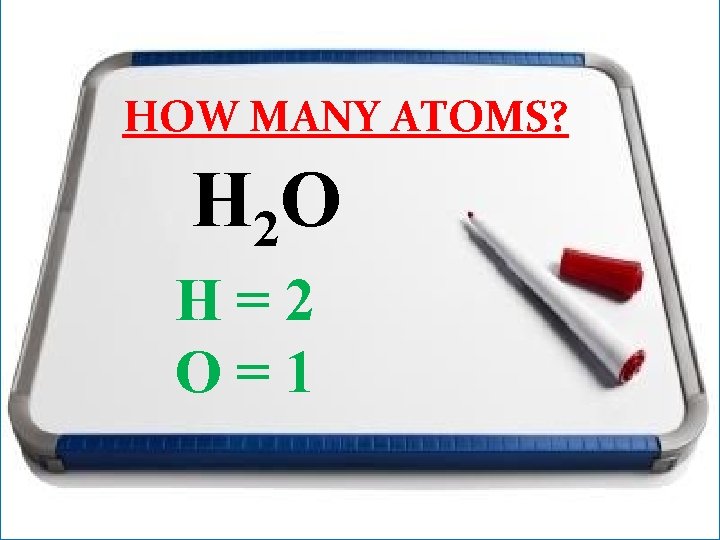
HOW MANY ATOMS? H 2 O

HOW MANY ATOMS? H 2 O H= O=

HOW MANY ATOMS? H 2 O H=2 O=1

HOW MANY ATOMS? SO 3

HOW MANY ATOMS? SO 3 S= O=

HOW MANY ATOMS? SO 3 S=1 O=3

HOW MANY ATOMS? H 2 SO 4

HOW MANY ATOMS? H 2 SO 4 H=2 S=1 O=4

HOW MANY ATOMS? Na. HCO 3

HOW MANY ATOMS? Na. HCO 3 Na = 1 H=1 C=1 O=3

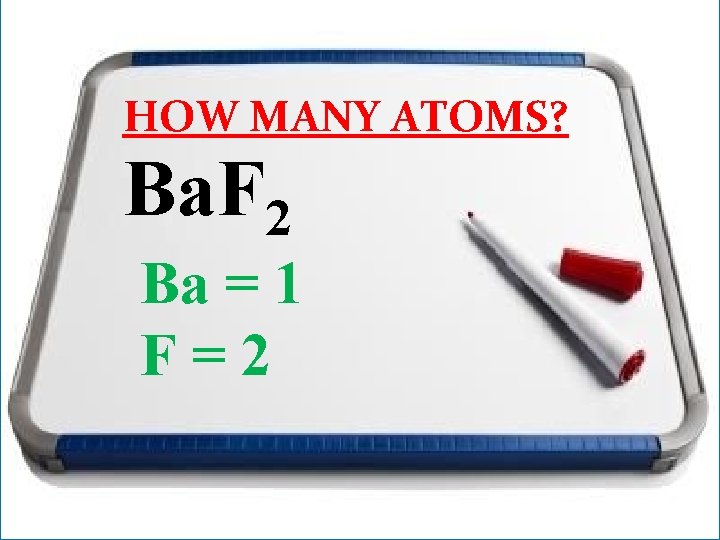
HOW MANY ATOMS? Ba. F 2

HOW MANY ATOMS? Ba. F 2 Ba = 1 F=2

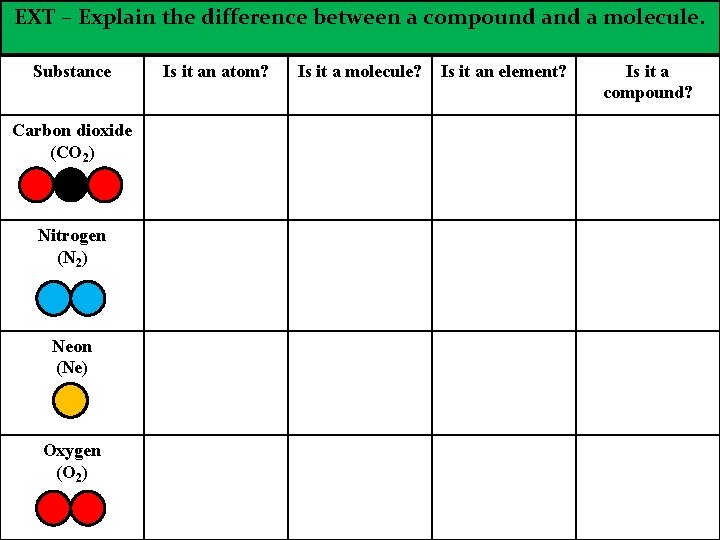
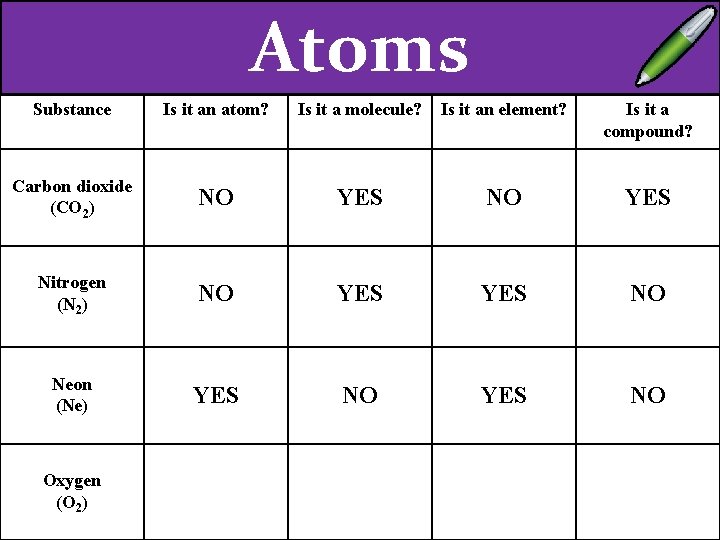
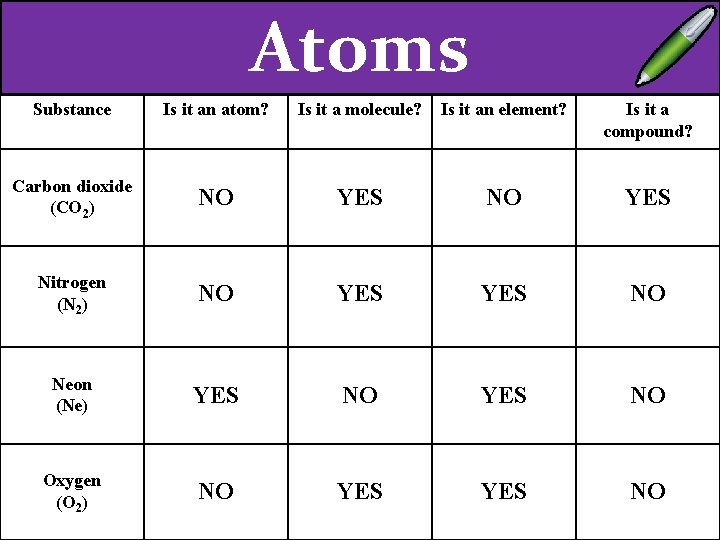
EXT – Explain the difference between a compound a molecule. Substance Carbon dioxide (CO 2) Nitrogen (N 2) Neon (Ne) Oxygen (O 2) Is it an atom? Is it a molecule? Is it an element? Is it a compound?

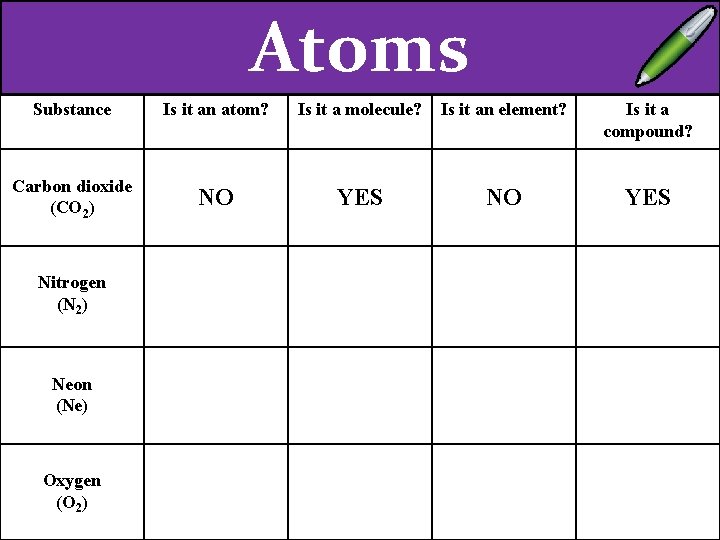
Atoms Substance Is it an atom? Is it a molecule? Is it an element? Is it a compound? Carbon dioxide (CO 2) NO YES Nitrogen (N 2) Neon (Ne) Oxygen (O 2)

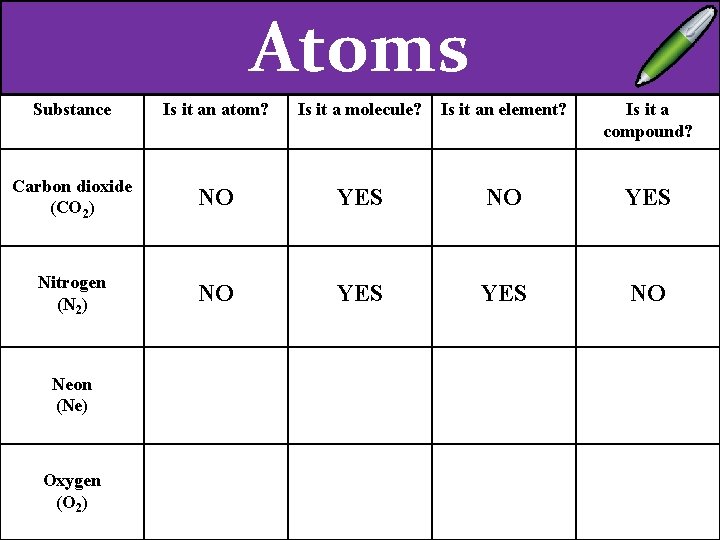
Atoms Substance Is it an atom? Is it a molecule? Is it an element? Is it a compound? Carbon dioxide (CO 2) NO YES Nitrogen (N 2) NO YES NO Neon (Ne) Oxygen (O 2)

Atoms Substance Is it an atom? Is it a molecule? Is it an element? Is it a compound? Carbon dioxide (CO 2) NO YES Nitrogen (N 2) NO YES NO Neon (Ne) YES NO Oxygen (O 2)

Atoms Substance Is it an atom? Is it a molecule? Is it an element? Is it a compound? Carbon dioxide (CO 2) NO YES Nitrogen (N 2) NO YES NO Neon (Ne) YES NO Oxygen (O 2) NO YES NO

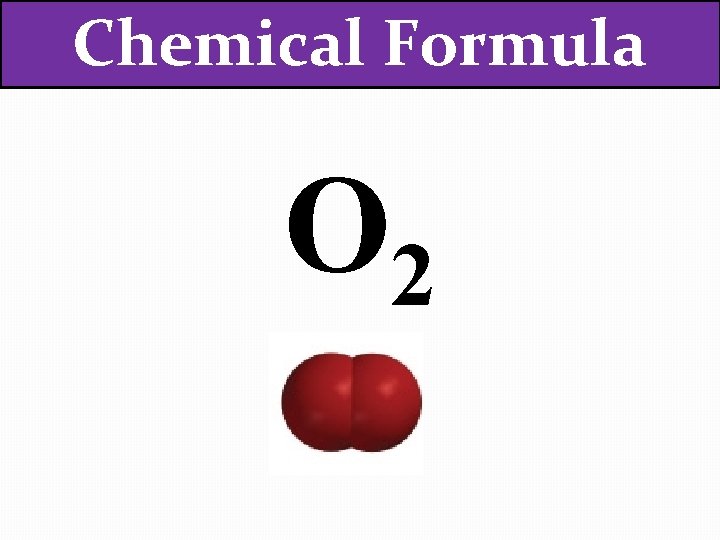
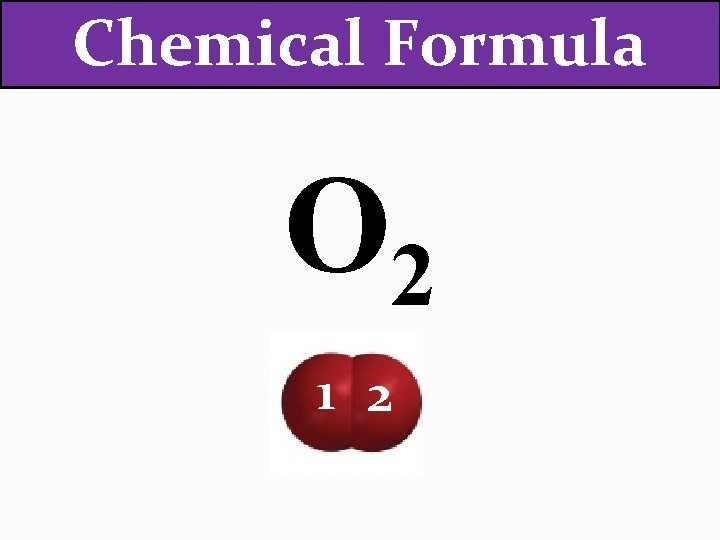
Chemical Formula O 2

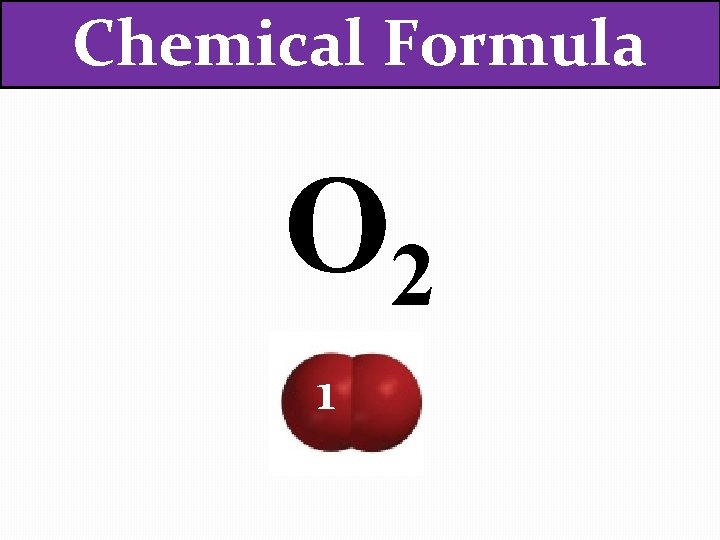
Chemical Formula O 2 1

Chemical Formula O 2 1 2

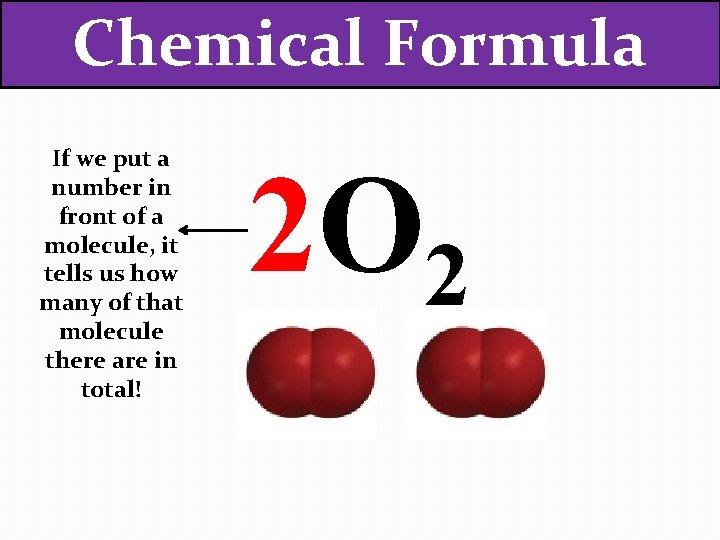
Chemical Formula If we put a number in front of a molecule, it tells us how many of that molecule there are in total! 2 O 2

Chemical Formula If we put a number in front of a molecule, it tells us how many of that molecule there are in total! 2 O 2 How many oxygen atoms are there now?

Chemical Formula If we put a number in front of a molecule, it tells us how many of that molecule there are in total! 2 O 2 1 How many oxygen atoms are there now?

Chemical Formula If we put a number in front of a molecule, it tells us how many of that molecule there are in total! 2 O 2 1 2 How many oxygen atoms are there now?

Chemical Formula If we put a number in front of a molecule, it tells us how many of that molecule there are in total! 2 O 2 1 2 3 How many oxygen atoms are there now?

Chemical Formula If we put a number in front of a molecule, it tells us how many of that molecule there are in total! 2 O 2 1 2 3 4 How many oxygen atoms are there now?

Chemical Formula If we put a number in front of a molecule, it tells us how many of that molecule there are in total! 2 O 2 1 2 3 4 How many oxygen atoms are there now?

Chemical Formula CO 2

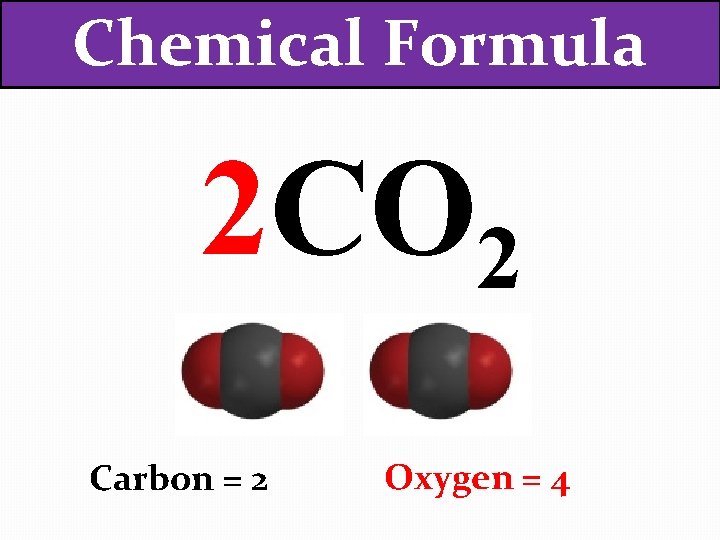
Chemical Formula 2 CO 2 How many of each atom are there now?

Chemical Formula 2 CO 2 Carbon = 2 Oxygen = 4

HOW MANY ATOMS? 3 H 2 O

HOW MANY ATOMS? 3 H 2 O H=6 O=3

HOW MANY ATOMS? 2 SO 3

HOW MANY ATOMS? 2 SO 3 S=2 O=6

HOW MANY ATOMS? 4 H 2 SO 4

HOW MANY ATOMS? 4 H 2 SO 4 H=8 S=4 O = 16

HOW MANY ATOMS? 3 Na. HCO 3

HOW MANY ATOMS? 3 Na. HCO 3 Na = 3 H=3 C=3 O=9

HOW MANY ATOMS? 5 Ba. F 2

HOW MANY ATOMS? 5 Ba. F 2 Ba = 5 F = 10

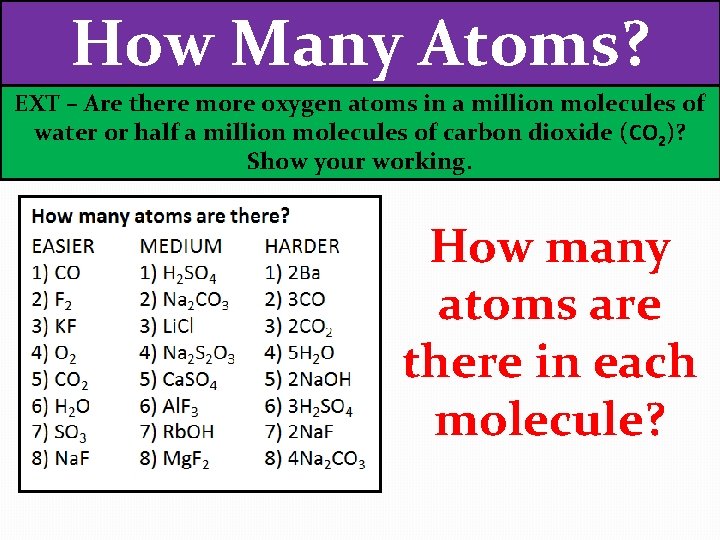
How Many Atoms? EXT – Are there more oxygen atoms in a million molecules of water or half a million molecules of carbon dioxide (CO 2)? Show your working. How many atoms are there in each molecule?

Atoms! EXT – Write the correct version of each false answer! Atoms are tiny particles. Atoms can be seen with a magnifying glass. Atoms make up everything around us. Atoms can be split into smaller units. Matter is the name given to all the objects and materials around us. 6. Atoms are all the same. 7. Indivisible means that something can be separated. 1. 2. 3. 4. 5.

Talk for 30 seconds! End