ATOMS Chapter Fourteen Atoms 14 1 The Structure




























- Slides: 41

ATOMS

Chapter Fourteen: Atoms Ø 14. 1 The Structure of the Atom Ø 14. 2 Electrons

Chapter 14. 1 Learning Goals ØIdentify and describe particles which comprise atoms. ØDescribe the effects of radioactivity. ØCompare and contrast forces inside atoms

Investigation 14 A Atomic Structure ØKey Question: What is inside an atom?

14. 1 Structure of the Atom ØIn order to understand atoms, we need to understand the idea of electric charge. ØWe know of two different kinds of electric charge and we call them positive and negative.

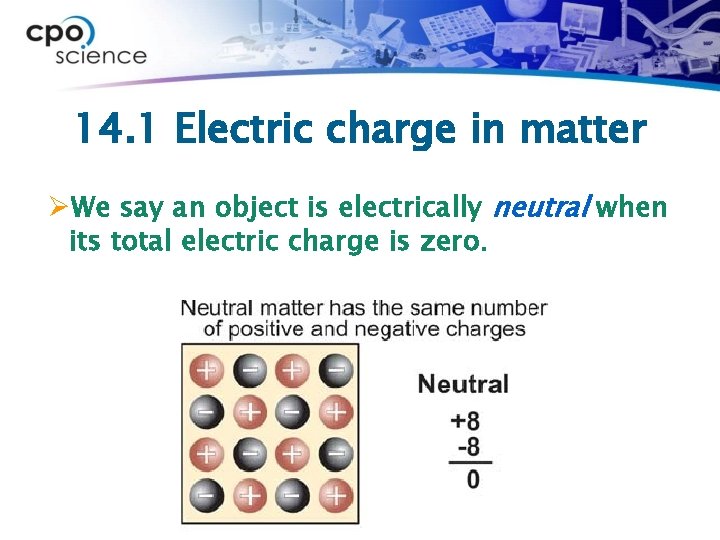
14. 1 Electric charge in matter ØWe say an object is electrically neutral when its total electric charge is zero.


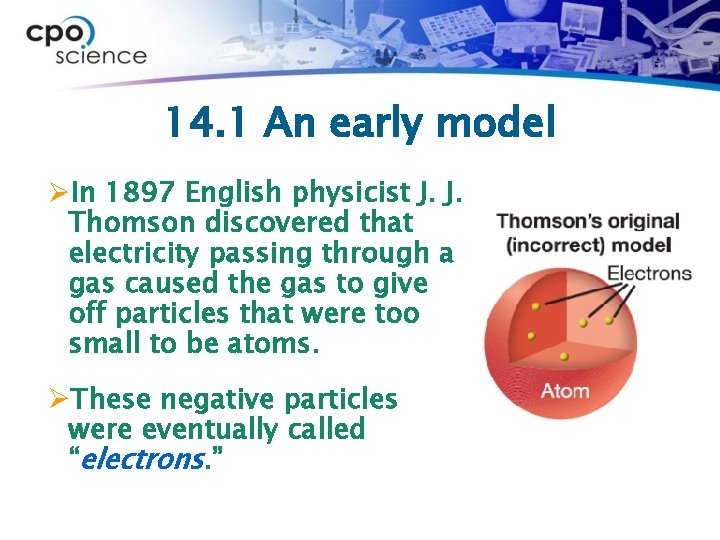
14. 1 An early model ØIn 1897 English physicist J. J. Thomson discovered that electricity passing through a gas caused the gas to give off particles that were too small to be atoms. ØThese negative particles were eventually called “electrons. ”

14. 1 The nuclear model ØIn 1911, Ernest Rutherford, Hans Geiger, and Ernest Marsden did a clever experiment to test Thomson’s model. ØWe now know that every atom has a tiny nucleus, which contains more than 99% of the atom’s mass.

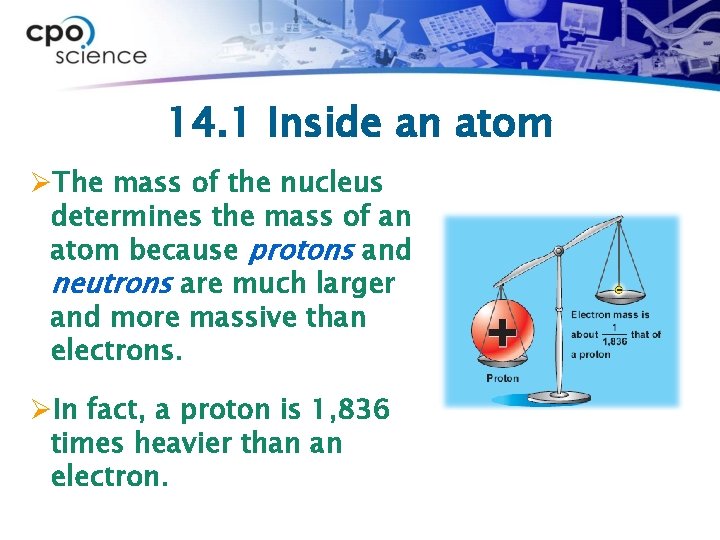
14. 1 Inside an atom ØThe mass of the nucleus determines the mass of an atom because protons and neutrons are much larger and more massive than electrons. ØIn fact, a proton is 1, 836 times heavier than an electron.



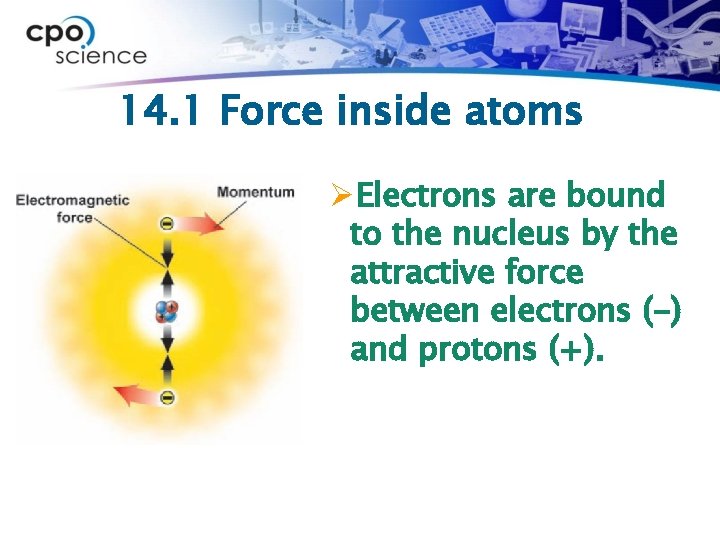
14. 1 Force inside atoms ØElectrons are bound to the nucleus by the attractive force between electrons (-) and protons (+).

14. 1 Force inside atoms ØWhat holds the nucleus together? ØThere is another force that is even stronger than the electric force. ØWe call it the strong nuclear force.

14. 1 How atoms of various elements are different ØThe atoms of different elements contain different numbers of protons in the nucleus. ØBecause the number of protons is so important, it is called the atomic number.

14. 1 How atoms of various elements are different ØIsotopes are atoms of the same element that have different numbers of neutrons. How are these carbon isotopes different? ØThe mass number of an isotope tells you the number of protons plus the number of neutrons.

14. 1 Radioactivity ØAlmost all elements have one or more isotopes that are stable. Ø“Stable” means the nucleus stays together. ØCarbon-14 is radioactive because it has an unstable nucleus.

Solving Problems ØHow many neutrons are present in an aluminum atom that has an atomic number of 13 and a mass number of 27?

Solving Problems 1. Looking for: Ø …number of neutrons in aluminum-27 2. Given Ø … atomic no. = 13; mass no. = 27 3. Relationships: Ø Periodic table says atomic no. = proton no. Ø protons + neutrons = mass no. 4. Solution Ø neutrons = mass no. – protons Ø neutrons = 27 – 13 = 14


Chapter Fourteen: Atoms Ø 14. 1 The Structure of the Atom Ø 14. 2 Electrons

Chapter 14. 2 Learning Goals ØCompare spectra of elements. ØExplain the Bohr atom model. ØApply principles of quantum theory to explain the behavior of electrons in atoms.

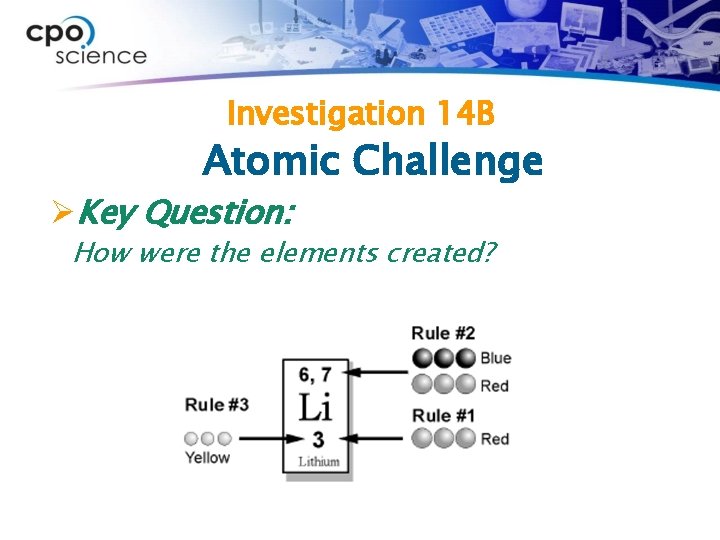
Investigation 14 B Atomic Challenge ØKey Question: How were the elements created?

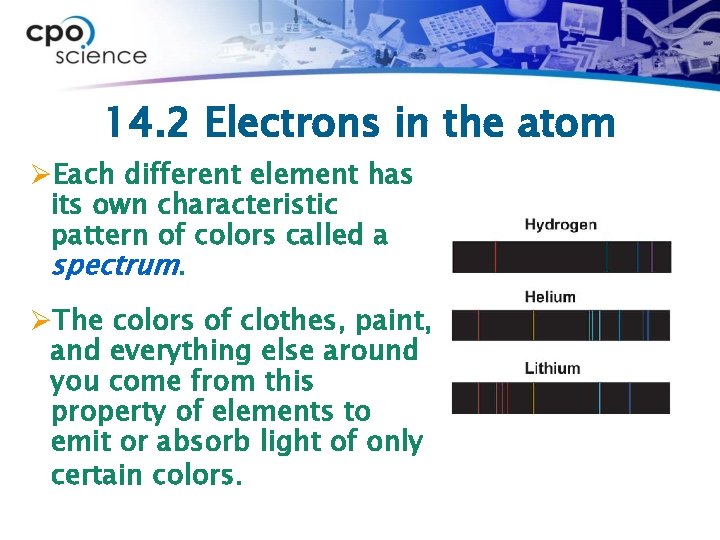
14. 2 Electrons in the atom ØEach different element has its own characteristic pattern of colors called a spectrum. ØThe colors of clothes, paint, and everything else around you come from this property of elements to emit or absorb light of only certain colors.

14. 2 Electrons in atoms ØEach individual color in a spectrum is called a spectral line because each color appears as a line in a spectroscope. ØA spectroscope is a device that spreads light into its different colors.


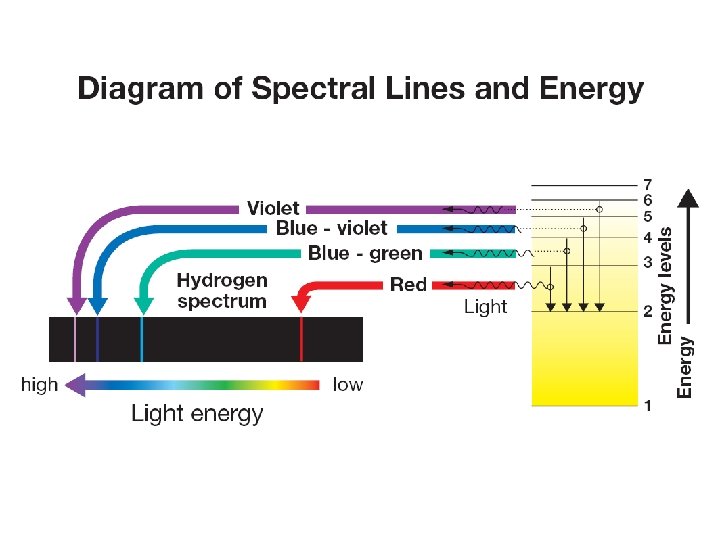
14. 2 Bohr model of the atom Ø Danish physicist Neils Bohr proposed the concept of energy levels to explain the spectrum of hydrogen. Ø When an electron moves from a higher energy level to a lower one, the atom gives up the energy difference between the two levels. Ø The energy comes out as different colors of light.


14. 2 The quantum theory ØQuantum theory says that when things get very small, like the size of an atom, matter and energy do not obey Newton’s laws or other laws of classical physics.

14. 2 The quantum theory ØAccording to quantum theory, particles the size of electrons are fundamentally different ØAn electron appears in a wave-like “cloud and has no definite position.

14. 2 The quantum theory ØThe work of German physicist Werner Heisenberg (1901– 1976) led to Heisenberg’s uncertainty principle. ØThe uncertainty principle explains why a particle’s position, momentum or energy can never be precisely determined. ØThe uncertainty principle exists because measuring any variable disturbs the others in an unpredictable way.

14. 2 The uncertainty principle

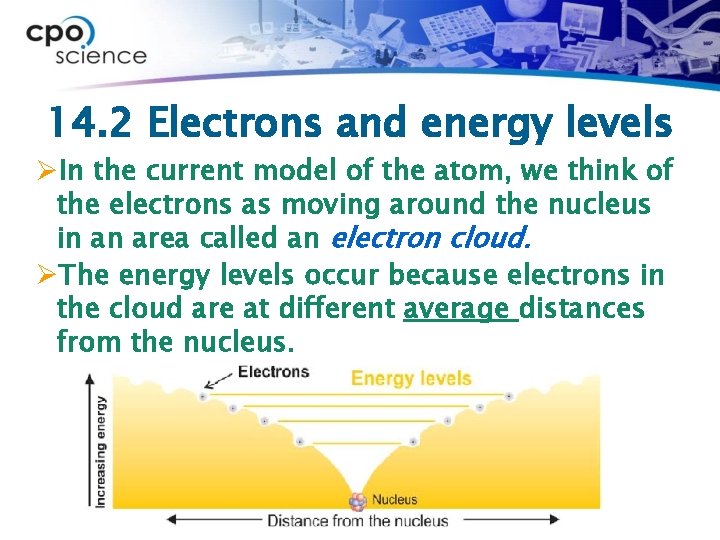
14. 2 Electrons and energy levels ØIn the current model of the atom, we think of the electrons as moving around the nucleus in an area called an electron cloud. ØThe energy levels occur because electrons in the cloud are at different average distances from the nucleus.

14. 2 Rules for energy levels Inside an atom, electrons always obey these rules: 1. The energy of an electron must match one of the energy levels in the atom. 2. Each energy level can hold only a certain number of electrons, and no more. 3. As electrons are added to an atom, they settle into the lowest unfilled energy level.

14. 2 Models of energy levels ØWhile Bohr’s model of electron energy levels explained atomic spectra and the periodic behavior of the elements, it was incomplete. ØEnergy levels are predicted by quantum mechanics, the branch of physics that deals with the microscopic world of atoms.


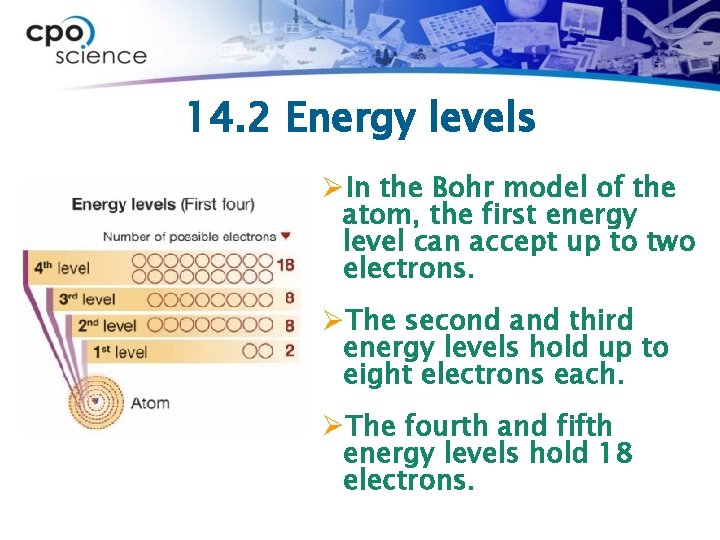
14. 2 Energy levels ØIn the Bohr model of the atom, the first energy level can accept up to two electrons. ØThe second and third energy levels hold up to eight electrons each. ØThe fourth and fifth energy levels hold 18 electrons.


14. 2 Electrons and energy levels Ø The first energy level can accept up to two electrons. Ø The second energy levels hold up to eight electrons.

Investigation 14 C Energy and Quantum Theory ØKey Question: How do atoms absorb and emit light energy?

Bioluminescence- Glow Live! Ø Like a glow stick, living things produce their own light using a chemical reaction. Bioluminescence is “cold light” because it doesn’t produce a lot of heat. While it takes a lot of energy for a living thing to produce light, almost 100 percent of the energy becomes visible light.
 Compared to atoms of metals, atoms of nonmetals generally
Compared to atoms of metals, atoms of nonmetals generally Chapter 4 section 2 the structure of atoms answer key
Chapter 4 section 2 the structure of atoms answer key Chapter 6 electronic structure of atoms answers
Chapter 6 electronic structure of atoms answers Electronic structure of atoms
Electronic structure of atoms 14 line iambic pentameter poem
14 line iambic pentameter poem A 14 line lyric poem
A 14 line lyric poem Eleven twelve thirteen fourteen fifteen
Eleven twelve thirteen fourteen fifteen Treaty of versailles vs wilson's 14 points
Treaty of versailles vs wilson's 14 points Perang dunia dan kelembagaan dunia
Perang dunia dan kelembagaan dunia Octave and sestet are part of
Octave and sestet are part of The diagram below represents the placoderm fish
The diagram below represents the placoderm fish What is poetry in literature
What is poetry in literature Siya ang may akda ng fourteen points
Siya ang may akda ng fourteen points Fourteen line lyric poem
Fourteen line lyric poem A 14 line lyric poem
A 14 line lyric poem Siya ang may akda ng fourteen points
Siya ang may akda ng fourteen points 14 line lyric poem
14 line lyric poem Teoryang klasismo halimbawa
Teoryang klasismo halimbawa Fourteen one act play summary
Fourteen one act play summary What are the fourteen principles of management
What are the fourteen principles of management Fourteen points and treaty of versailles similarities
Fourteen points and treaty of versailles similarities Crosby's fourteen steps to quality improvement
Crosby's fourteen steps to quality improvement Space bar
Space bar Ap chemistry electronic structure of atoms
Ap chemistry electronic structure of atoms Quantum theory and the electronic structure of atoms
Quantum theory and the electronic structure of atoms Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Lp html
Lp html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Gấu đi như thế nào
Gấu đi như thế nào Tư thế worm breton
Tư thế worm breton Hát lên người ơi alleluia
Hát lên người ơi alleluia Các môn thể thao bắt đầu bằng tiếng chạy
Các môn thể thao bắt đầu bằng tiếng chạy Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Cong thức tính động năng
Cong thức tính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân 101012 bằng
101012 bằng độ dài liên kết
độ dài liên kết Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật