ATOMS Atom Smallest part of an element with


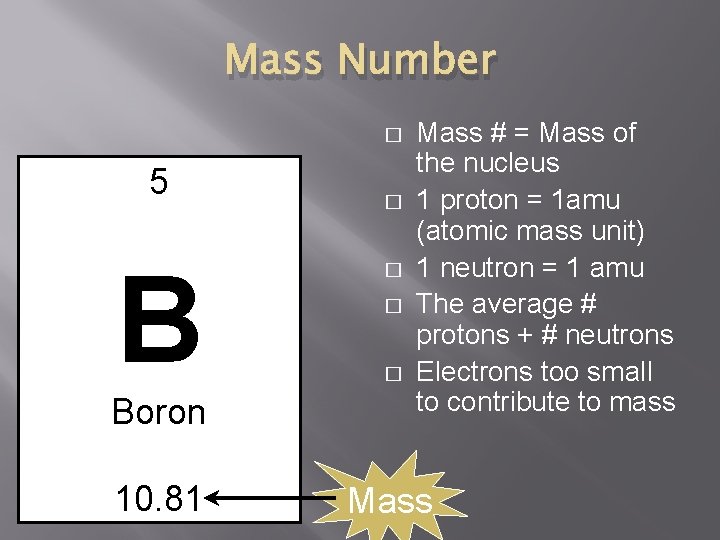



- Slides: 21

ATOMS

Atom � Smallest part of an element with all the properties of that element. � 2 parts

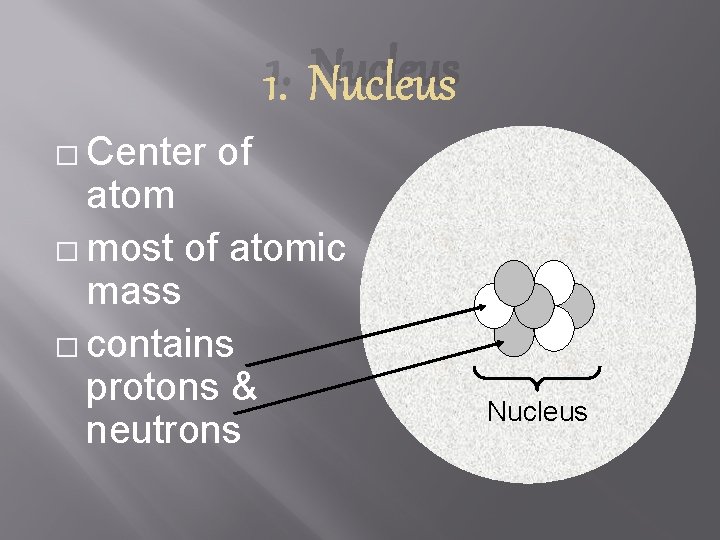
1. Nucleus � Center of atom � most of atomic mass � contains protons & neutrons Nucleus
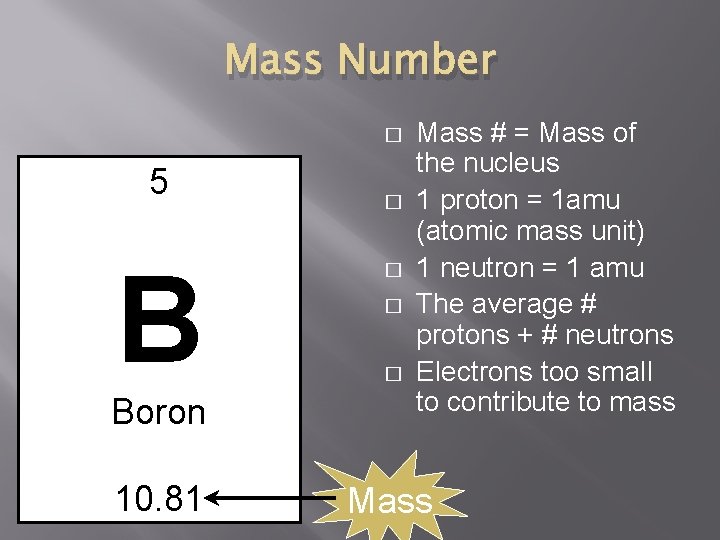
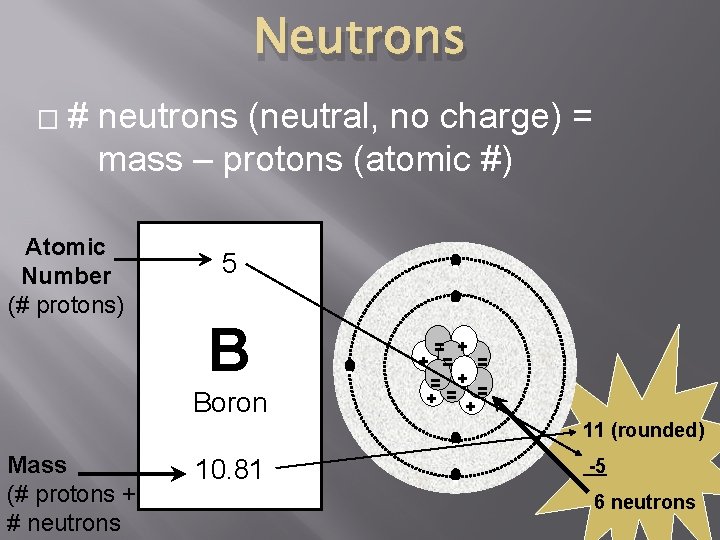
Mass Number � 5 B Boron 10. 81 � � Mass # = Mass of the nucleus 1 proton = 1 amu (atomic mass unit) 1 neutron = 1 amu The average # protons + # neutrons Electrons too small to contribute to mass Mass

What is the mass (rounded to the nearest whole number) of an atom of each of these elements? 11 24 36 Na Cr Kr Sodium 22. 99 Chromium 51. 99 Krypton 83. 80 A. 11 A. 24 A. 36 B. 23 Na = 23 C. 22. 99 B. 51 Cr = 52 C. 51. 99 B. 83 Kr = 84 C. 83. 80 D. 22 D. 52 D. 84

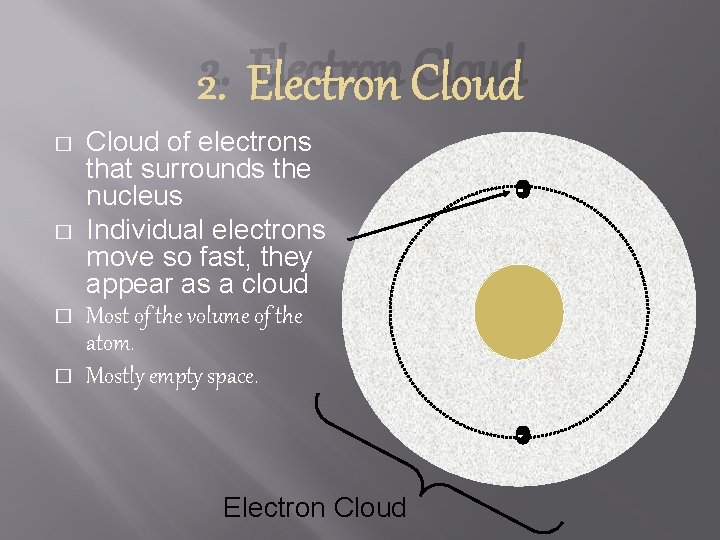
2. Electron Cloud � � Cloud of electrons that surrounds the nucleus Individual electrons move so fast, they appear as a cloud Most of the volume of the atom. Mostly empty space. - - Electron Cloud

3 subatomic particles 1. 2. 3. Protons: positive charge (+) located in nucleus Neutrons: neutral charge (=) located in nucleus Electrons: negative charge (-) located in the electron cloud

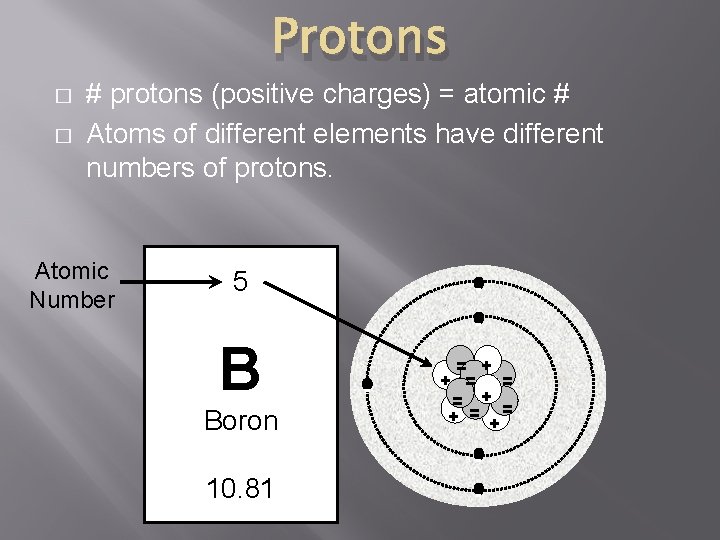
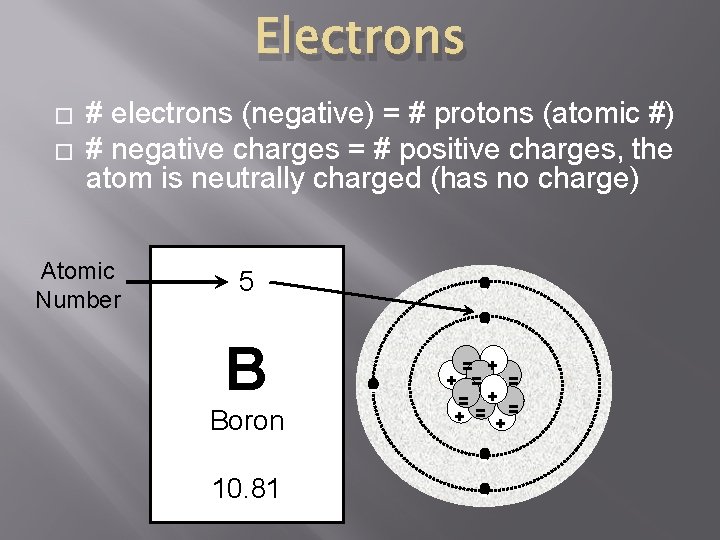
Protons � � # protons (positive charges) = atomic # Atoms of different elements have different numbers of protons. Atomic Number 5 - B Boron - = + + = = = + = + - 10. 81 -

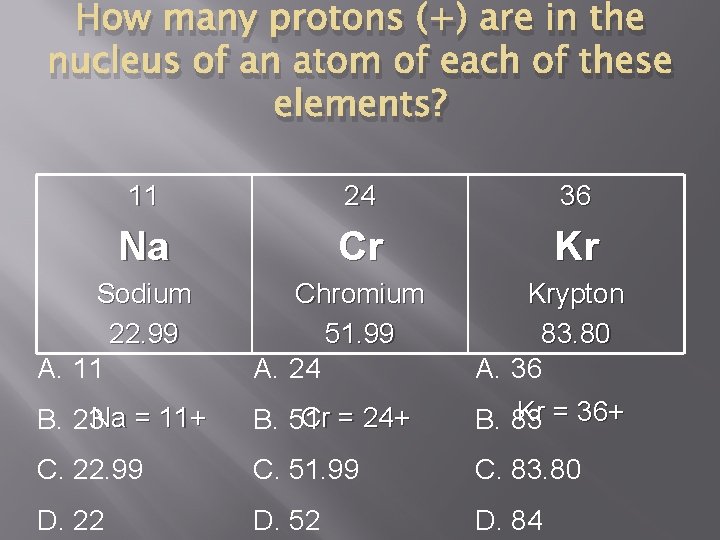
How many protons (+) are in the nucleus of an atom of each of these elements? 11 24 36 Na Cr Kr Sodium 22. 99 A. 11 Chromium 51. 99 A. 24 Krypton 83. 80 A. 36 B. 23 Na = 11+ Cr = 24+ B. 51 Kr = 36+ B. 83 C. 22. 99 C. 51. 99 C. 83. 80 D. 22 D. 52 D. 84

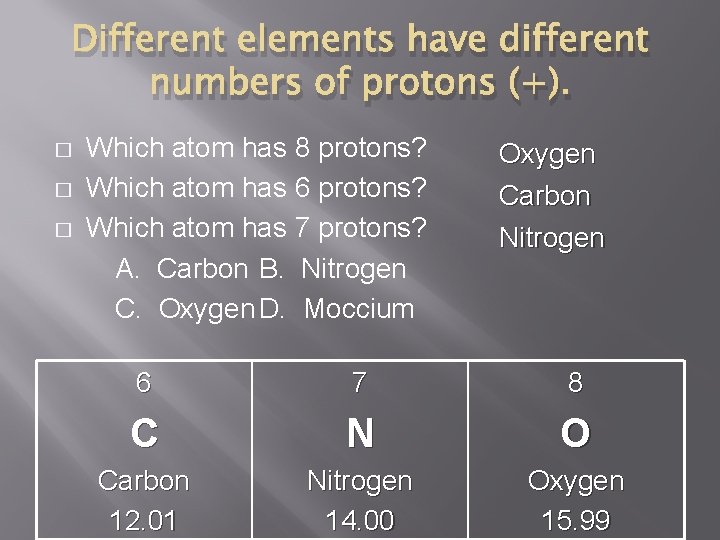
Different elements have different numbers of protons (+). � � � Which atom has 8 protons? Which atom has 6 protons? Which atom has 7 protons? A. Carbon B. Nitrogen C. Oxygen D. Moccium Oxygen Carbon Nitrogen 6 7 8 C N O Carbon 12. 01 Nitrogen 14. 00 Oxygen 15. 99

Neutrons � # neutrons (neutral, no charge) = mass – protons (atomic #) Atomic Number (# protons) 5 - B Boron Mass (# protons + # neutrons - - = + + = = = + = + - 10. 81 - 11 (rounded) -5 6 neutrons

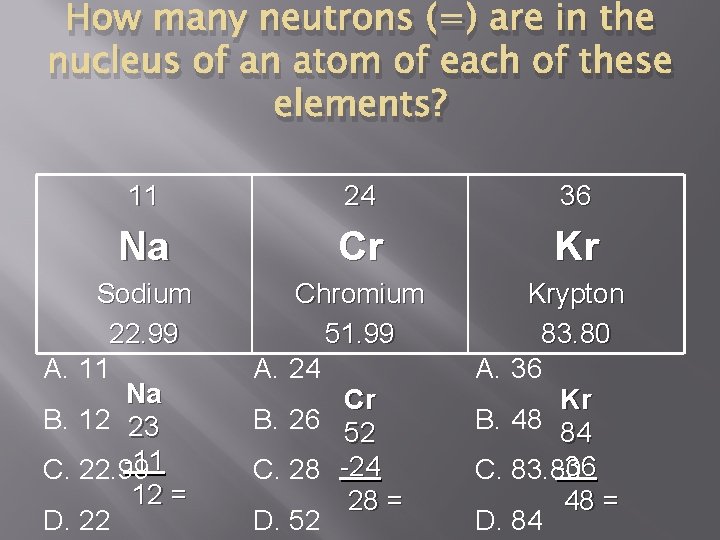
How many neutrons (=) are in the nucleus of an atom of each of these elements? 11 24 36 Na Cr Kr Sodium 22. 99 A. 11 Na B. 12 23 -11 C. 22. 99 12 = D. 22 Chromium 51. 99 A. 24 Cr B. 26 52 C. 28 -24 28 = D. 52 Krypton 83. 80 A. 36 Kr B. 48 84 -36 C. 83. 80 48 = D. 84

Electrons � � # electrons (negative) = # protons (atomic #) # negative charges = # positive charges, the atom is neutrally charged (has no charge) Atomic Number 5 - B Boron - = + + = = = + = + - 10. 81 -

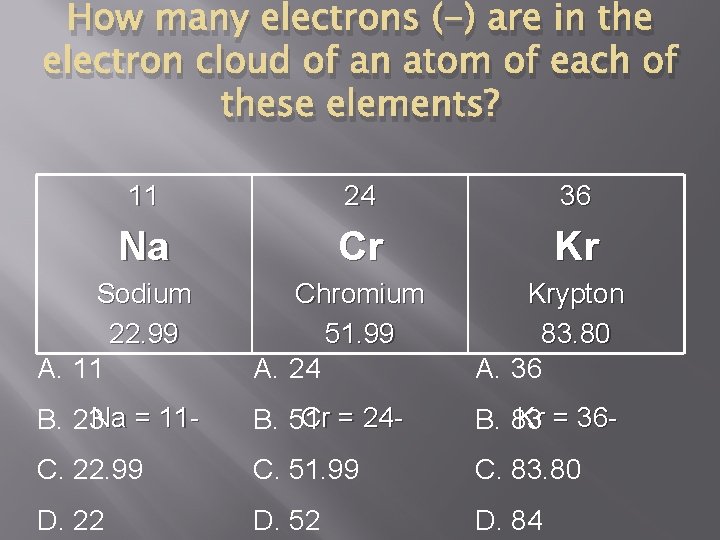
How many electrons (-) are in the electron cloud of an atom of each of these elements? 11 24 36 Na Cr Kr Sodium 22. 99 A. 11 Chromium 51. 99 A. 24 Krypton 83. 80 A. 36 B. 23 Na = 11 - Cr = 24 B. 51 Kr = 36 B. 83 C. 22. 99 C. 51. 99 C. 83. 80 D. 22 D. 52 D. 84

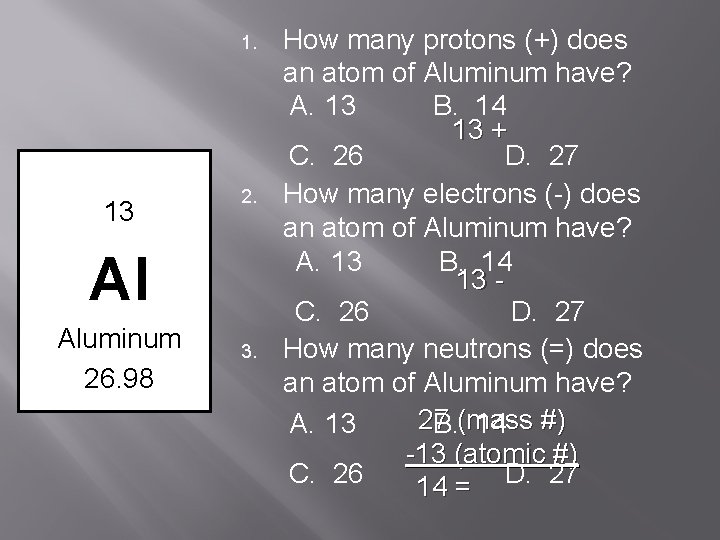
1. 13 2. Al Aluminum 26. 98 3. How many protons (+) does an atom of Aluminum have? A. 13 B. 14 13 + C. 26 D. 27 How many electrons (-) does an atom of Aluminum have? A. 13 B. 14 13 C. 26 D. 27 How many neutrons (=) does an atom of Aluminum have? 27 A. 13 B. (mass 14 #) -13 (atomic #) C. 26 D. 27 14 =

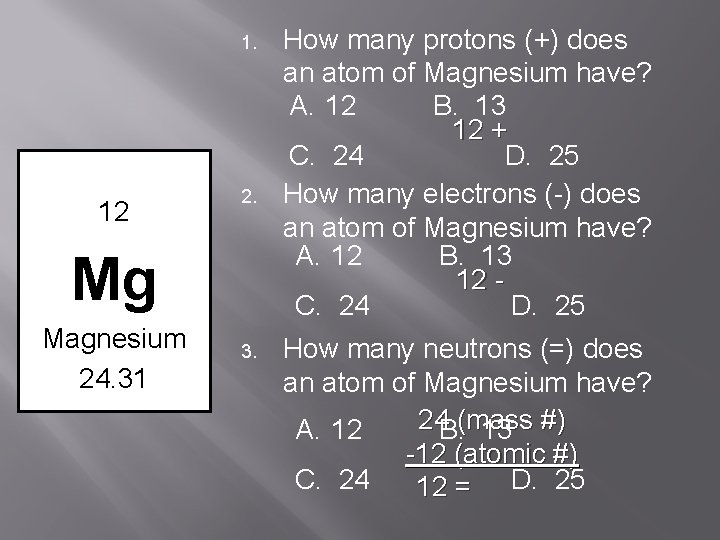
1. 12 2. Mg Magnesium 24. 31 3. How many protons (+) does an atom of Magnesium have? A. 12 B. 13 12 + C. 24 D. 25 How many electrons (-) does an atom of Magnesium have? A. 12 B. 13 12 C. 24 D. 25 How many neutrons (=) does an atom of Magnesium have? 24 B. (mass A. 12 13 #) -12 (atomic #) C. 24 12 = D. 25

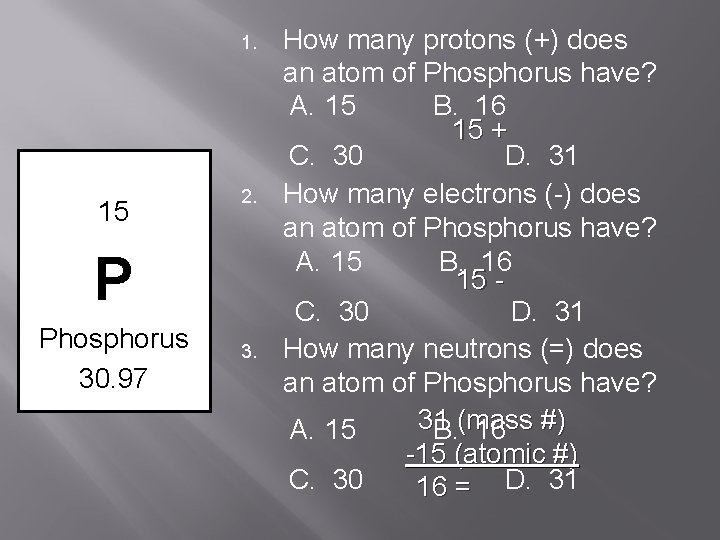
1. 15 2. P Phosphorus 30. 97 3. How many protons (+) does an atom of Phosphorus have? A. 15 B. 16 15 + C. 30 D. 31 How many electrons (-) does an atom of Phosphorus have? A. 15 B. 16 15 C. 30 D. 31 How many neutrons (=) does an atom of Phosphorus have? 31 A. 15 B. (mass 16 #) -15 (atomic #) C. 30 16 = D. 31

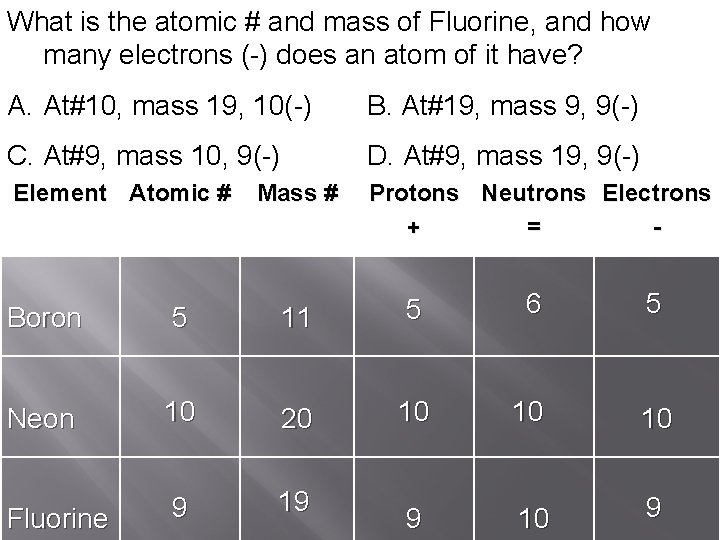
How many What is theprotons atomic #(+), of neutrons and Neon, massand of(=), Fluorine, how and many electrons and protons how(-) (+) doesneutrons and many an electrons atom (=) of(-) Boron doesan have? anatomofofit ithave? Fill in the table below. A. At#10, 5 (+), 5(=), 20(+), mass 5(-) 19, 10(=) 10(-) B. At#19, At#10, 11(+), mass 10(+), 11(=), 9, 10(=) 11(-) 9(-) C. At#9, 5 (+), mass At#20, 6(=), 10(+), 5(-) 10, 10(=) 9(-) D. At#9, At#10, 5(+), mass 11(=), 10(+), 19, 5(-) 20(=) 9(-) Element Atomic # Protons Neutrons Electrons + = - Mass # Boron 5 11 5 6 5 Neon 10 20 10 10 10 Fluorine 9 19 9 10 9

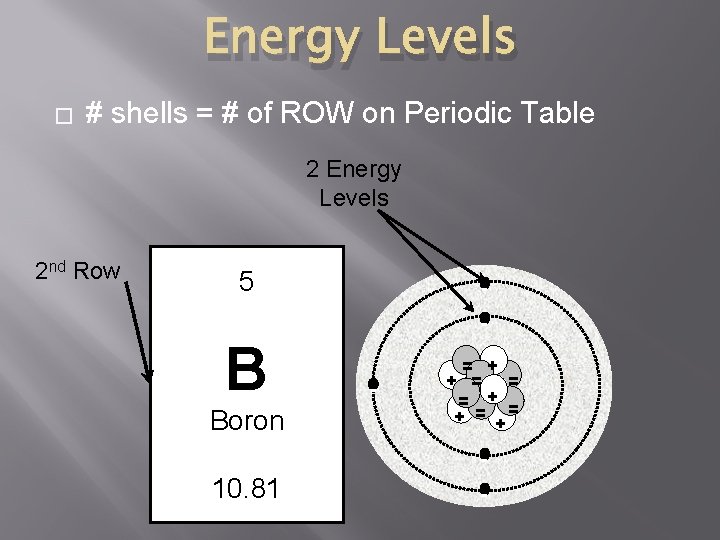
Energy Levels � # shells = # of ROW on Periodic Table 2 Energy Levels 2 nd Row 5 - B Boron - = + + = = = + = + - 10. 81 -

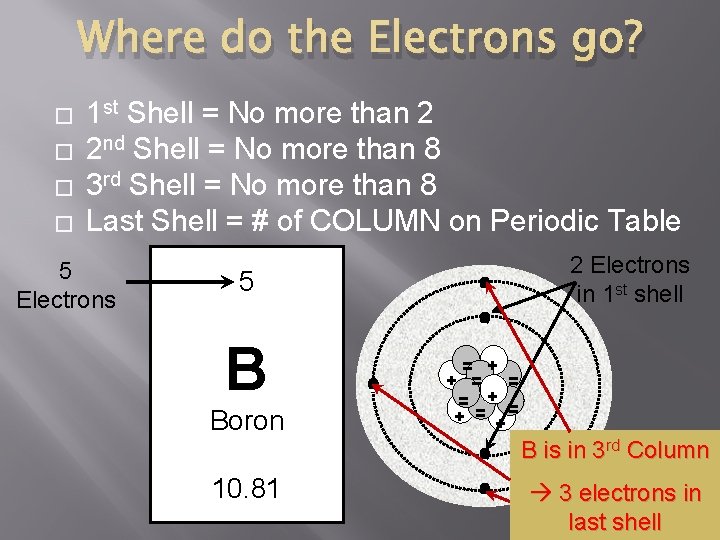
Where do the Electrons go? � � 1 st Shell = No more than 2 2 nd Shell = No more than 8 3 rd Shell = No more than 8 Last Shell = # of COLUMN on Periodic Table 5 Electrons 5 - 2 Electrons in 1 st shell - B Boron - = + + = = = + = + - 10. 81 - B is in 3 rd Column 3 electrons in last shell

Diagram Atoms 1 -18 � � � Teacher demonstrates diagramming atoms with atomic #1, #2, #3, #10, #18 Students work with partners – one sheet of paper team. Take turns diagramming the atoms. While one draws, the other checks work. One person diagrams the odd numbered elements, partner checks work. The other person diagrams the even numbered elements, partner checks work.