Atoms and Subatomic Particles Ch 4 Section 2
Atoms and Subatomic Particles Ch 4. Section 2

How big is an atom? ► An atom is incredibly small § The diameter of an atom would have to be increased 200 million times to have the diameter of a penny § If an apple were enlarged to the size of the Earth, the atoms in the apple would be the size of cherries
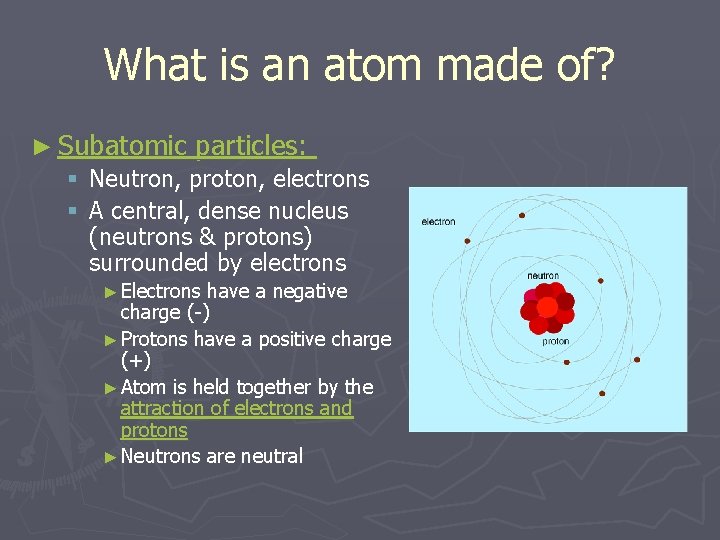
What is an atom made of? ► Subatomic particles: § Neutron, proton, electrons § A central, dense nucleus (neutrons & protons) surrounded by electrons ► Electrons have a negative charge (-) ► Protons have a positive charge (+) ► Atom is held together by the attraction of electrons and protons ► Neutrons are neutral

How much does an atom weigh? (What is it’s mass? ) ► Atoms are so small that it is impractical to use grams § 1 atom 10 -23 g ► Instead atoms are measured in atomic mass units or amu’s § One carbon atom is 12. 0 amu’s and all atoms are compared to this
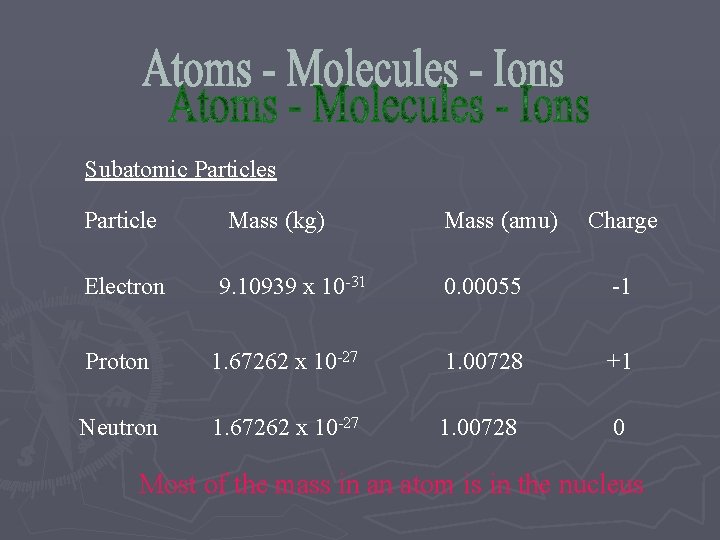
Subatomic Particles Particle Electron Mass (kg) 9. 10939 x 10 -31 Mass (amu) Charge 0. 00055 -1 Proton 1. 67262 x 10 -27 1. 00728 +1 Neutron 1. 67262 x 10 -27 1. 00728 0 Most of the mass in an atom is in the nucleus
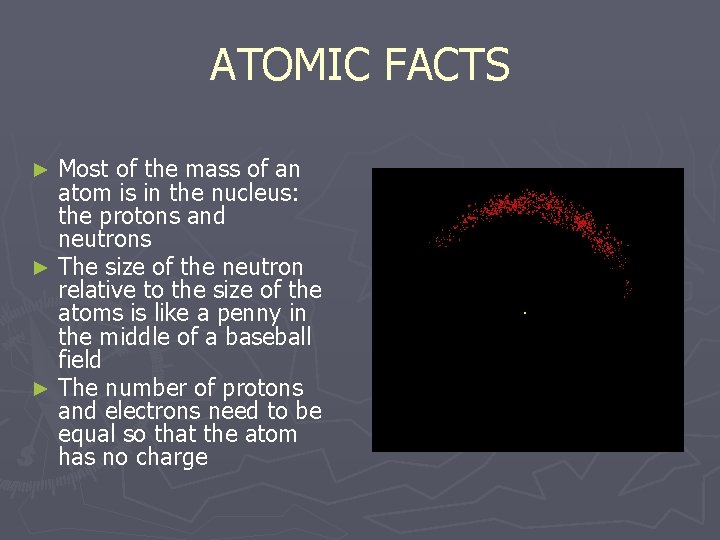
ATOMIC FACTS Most of the mass of an atom is in the nucleus: the protons and neutrons ► The size of the neutron relative to the size of the atoms is like a penny in the middle of a baseball field ► The number of protons and electrons need to be equal so that the atom has no charge ►
ATOMIC NUMBER ► The number of protons in the nucleus of an atom § Atomic numbers of naturally occurring elements: ►Lowest=Hydrogen § Z=1 ►Highest=Uranium § Z=92 ► Periodic (H) (U) Table of Elements
ATOMIC NUMBER Remember, § The number of protons in the nucleus of an atom equal the number electrons so, ►Atomic number= ►Number of protons= ►Number of electrons

Mass Number ► Sum of the number of protons and the number of neutrons in the nucleus of the atom: § Mass number=# of protons + # of neutrons § And…. . ►Mass number=atomic number + # of neutrons ►# of neutrons=mass number – atomic number
Isotopes ► Atoms of an element that have the same number of protons and electrons but different numbers of neutrons. § Hydrogen isotopes ►Hydrogen has 1 proton, 1 electron and 0 neutrons ►Deuterium has 1 proton, 1 electron and 1 neutron § Therefore, it is heavier than hydrogen but has similar chemical properties and slightly different physical properties ►Tritium has 1 proton, 1 electron and 2 neutrons

Atomic Masses ► Atomic mass Mass number § ► They are close but not the same Here’s why 1. Mass number is an exact number & atomic mass is a weight so it’s an inexact number Hydrogen has a mass number of 1 (1 protron + 0 neutrons), ► But weighs: 1. 00728 amu ► 2. Atomic masses are calculated based on the percentage of isotopes present in natural world § § § H=99. 985%; D=. 015%; T=trace Atomic mass of H is: 1. 00794 Periodic table of elements
Chemical Basis of Life ► Most common elements in living organisms § Carbon (C): 9. 5% § Hydrogen (H): 63% § Oxygen (O): 25. 5% § Nitrogen (N): 1. 4%

Macrominerals ► Minerals we need a lot of § Calcium (Ca) § Phosphorous (P) § Magnesium (Mg) § Potassium (K) § Sodium (Na)

Microminerals ► Needed in small quantities § Iron (Fe) § Copper (Cu) § Chromium (Cr) § Fluoride (F) § Iodine (I) § Selenium (Se) § Molybdenum (Mo) § Zinc (Zn)
- Slides: 14