Atoms and Molecules Observation Salt Grains Question What

Atoms and Molecules

Observation: Salt Grains ü Question: What makes up a single grain of salt? ü Observation: 1. Using a magnifying glass, carefully observe a salt grain. 2. Write down your observations and 1 -2 questions you have about this phenomena.


Did you know?

Guiding Question üHow are the smaller particles (atoms) of matter organized to make up everything in the universe?

Video: Atoms and Molecules https: //www. youtube. com/watch? v=vl. SOESXQI 7 o

Class Discussion Questions: 1. What are the particles called? 2. Do these particles make up everything we see around us? 3. What is everything that we see around us called? 4. What can be said about the size of the particles? 5. Are there many different types of particles?

What is the size of an atom? Just How Small is an Atom? https: //www. youtube. com/watch? v=y. QP 4 UJh. Nn 0 I&feature=youtu. be How Small is an Atom? (if time permits) https: //www. youtube. com/watch? v=mv. Uhr. Qrtr. BU&feature=youtu. be
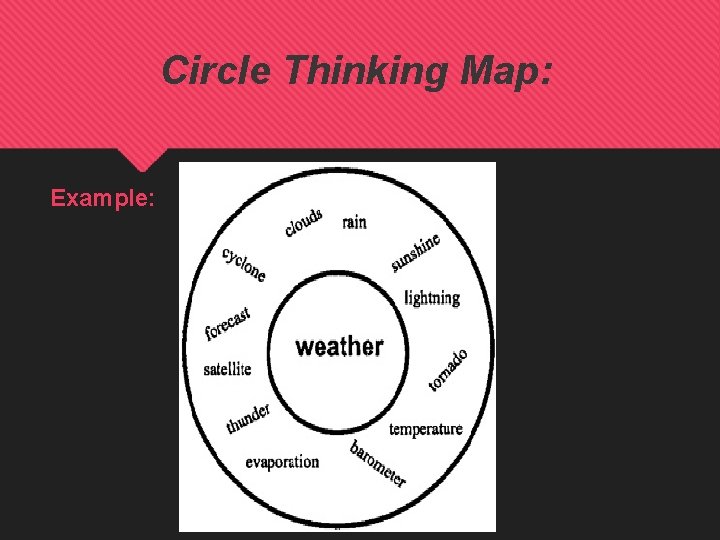
Circle Thinking Map: Example:

Circle Map: ATOMS

ELEMENTS 1. Examine the periodic table. 2. Make a list of any elements you are familiar with or have heard of before today. 3. Share what you know about the elements you recognize!

ATOMS Atoms are the building blocks of all matter. An atom is made up of smaller bits called… Name Protons Charge Position Mass +ve Nucleus 1 Electrons -ve Outside 0 Neutrons neutral Nucleus 1
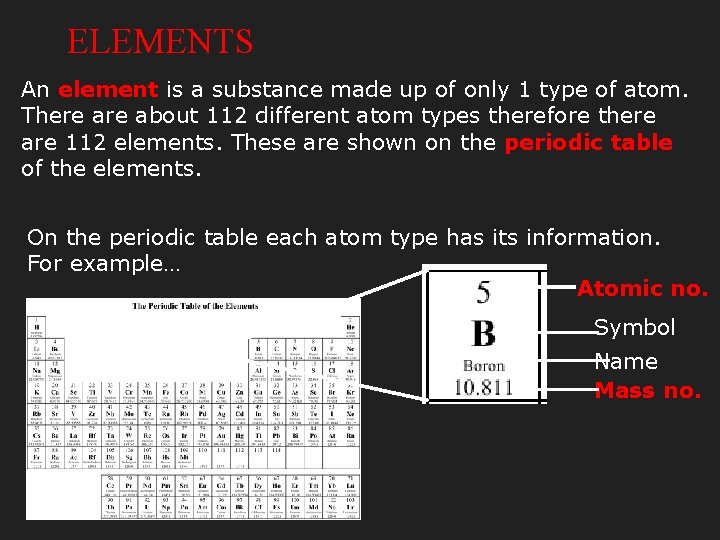
ELEMENTS An element is a substance made up of only 1 type of atom. There about 112 different atom types therefore there are 112 elements. These are shown on the periodic table of the elements. On the periodic table each atom type has its information. For example… Atomic no. Symbol Name Mass no.
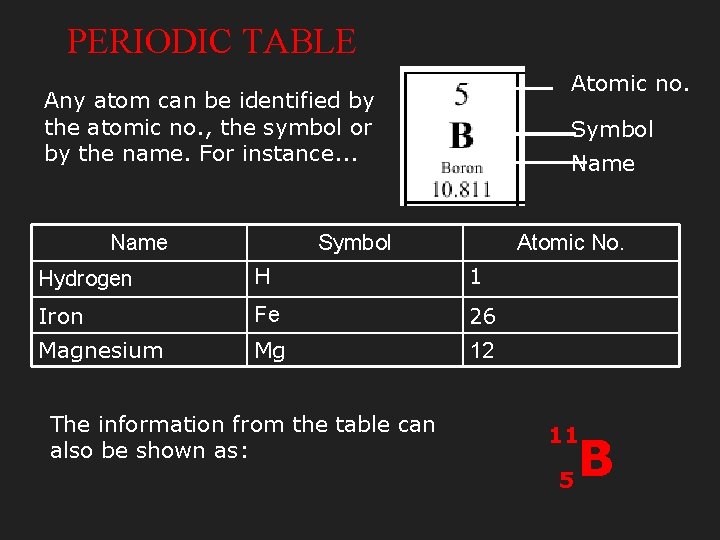
PERIODIC TABLE Atomic no. Any atom can be identified by the atomic no. , the symbol or by the name. For instance. . . Name Symbol Atomic No. Hydrogen H 1 Iron Fe 26 Magnesium Mg 12 The information from the table can also be shown as: 11 5 B
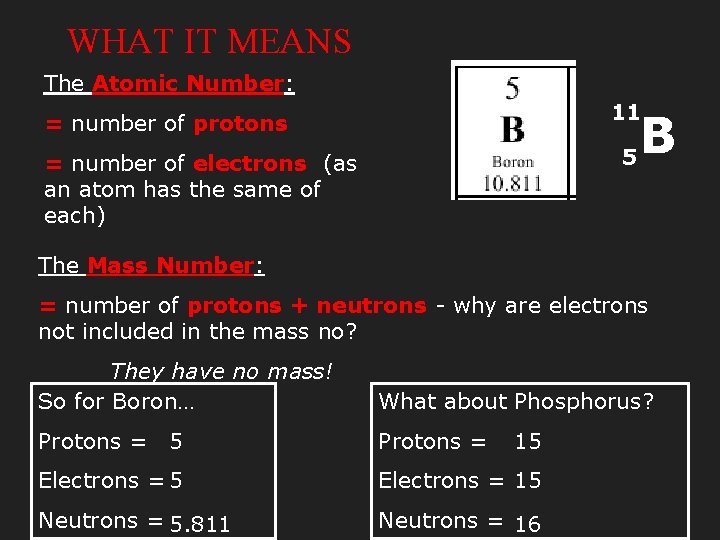
WHAT IT MEANS The Atomic Number: 11 = number of protons 5 = number of electrons (as an atom has the same of each) B The Mass Number: = number of protons + neutrons - why are electrons not included in the mass no? They have no mass! So for Boron… What about Phosphorus? Protons = 5 Protons = Electrons = 5 Electrons = 15 Neutrons = 5. 811 Neutrons = 16 15

Learning Check Select the correct symbol for each: A. Calcium 1) C 2) Ca 3) CA B. Sulfur 1) S 2) Sl C. Iron 1) Ir 2) FE 3) Fe 3) Su

Learning Check Select the correct name for each: A. N 1)neon 2)nitrogen 3)nickel B. P 1)potassium 2)phlogiston 3)phosphorus C. Ag 1) silver 2)agean 3)gold
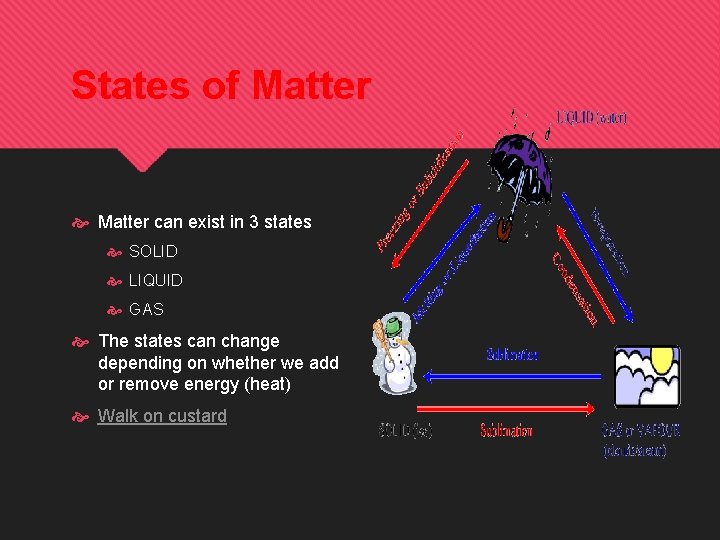
States of Matter can exist in 3 states SOLID LIQUID GAS The states can change depending on whether we add or remove energy (heat) Walk on custard
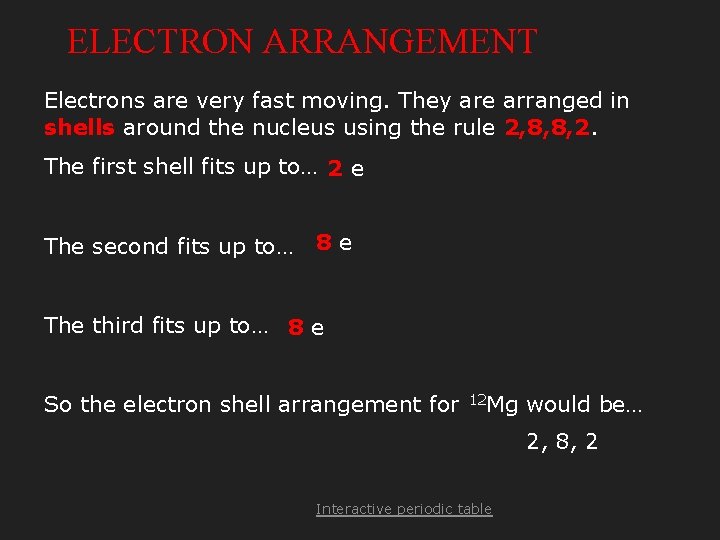
ELECTRON ARRANGEMENT Electrons are very fast moving. They are arranged in shells around the nucleus using the rule 2, 8, 8, 2. The first shell fits up to… 2 e The second fits up to… 8 e The third fits up to… 8 e So the electron shell arrangement for 12 Mg would be… 2, 8, 2 Interactive periodic table

GROUPS The columns in the periodic table are called groups. Groups of elements share similar reactivity. This is because they have the same number of valence electrons. As you go down a group the reactivity increases. Group 1 are the alkali metals properties: good conductors solid at room temperatures can be cut with a knife low densities and melting points Group 17 are the Halogens
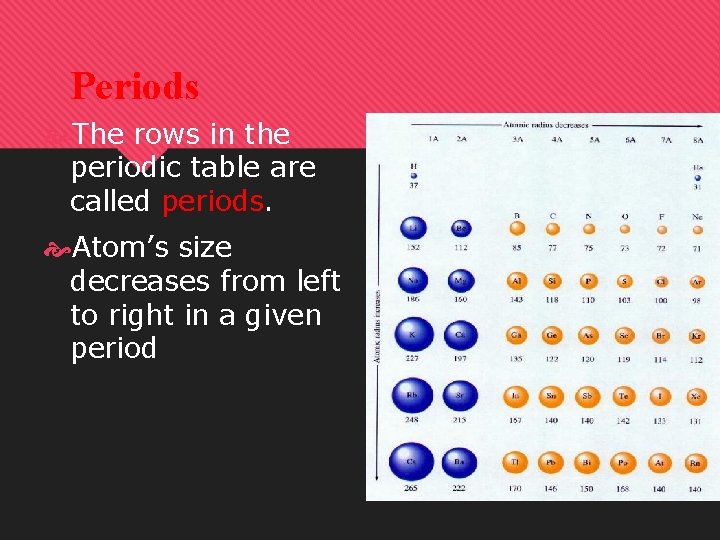
Periods The rows in the periodic table are called periods. Atom’s size decreases from left to right in a given period

Learning Check A. Element in Group XVII, Period 4 1) Br 2) Cl 3) Mn B. Element in Group II, Period 3 1) beryllium 2) magnesium 3) boron
Structure of the atom Nobel prize Chemistry 1908 Atomic model 1911 First person to transmute Nitrogen to oxygen 1919 $100 note

IONS An ion is an atom that has gained or lost electrons meaning it is negatively or positively charged. Atoms do this to get a full outer (valence) electron shell and so becomes more stable. The atom will get a full outer shell the simplest way it can, e. g. for 12 Mg: - Electron arrangement of 2, 8, 2 - it will LOSE 2 electrons (become 2, 8) - Now it has 10 electrons, but still has 12 protons. It has a 2+ charge. The ion is called Mg 2+. Superscript is used for ion charges
IONIC COMPOUNDS The ions that have been formed are now attracted to oppositely charged ions. So Mg 2+ will be attracted to Cl-. This forms an ionic compound. Some ionic compounds are soluble others are insoluble. Compounds which are insoluble form solids called precipitates. Lets do an experiment
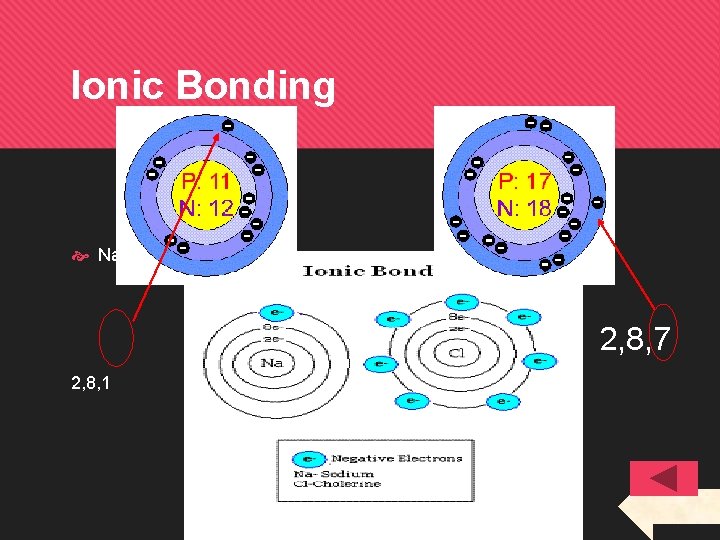
Ionic Bonding Na + Cl 2, 8, 7 2, 8, 1

IONIC FORMULAE So Mg 2+ will be attracted to Cl-. Because Mg is 2+ and Cl is only 1 -, Mg will attract 2 Cl’s. The subscript shows that they are 2 Cl’s for each Mg. If the starting ions were Cu 2+ and S 2 -, the 2 ions have the same charge. So each Cu will only attract 1 S. The compound formed will be Mg. Cl 2. The compound formed will be Cu. S. There is never any charges on the final product they balance out

Solubility If a solid compound dissolves in a solvent we say it is soluble. Not all solids will dissolve in water but some may dissolve in another type of liquid. Water is the universal solvent.
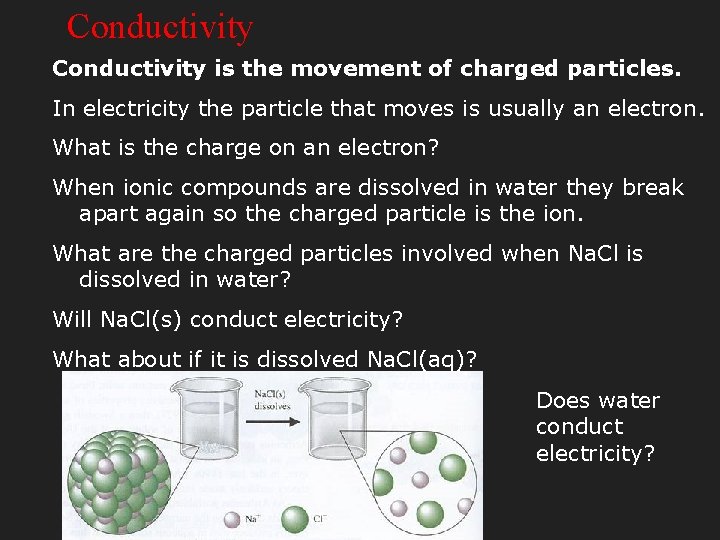
Conductivity is the movement of charged particles. In electricity the particle that moves is usually an electron. What is the charge on an electron? When ionic compounds are dissolved in water they break apart again so the charged particle is the ion. What are the charged particles involved when Na. Cl is dissolved in water? Will Na. Cl(s) conduct electricity? What about if it is dissolved Na. Cl(aq)? Does water conduct electricity?
Polymers Some compounds may form long chains of repeating units (monomers), these form polymers. Longer chains get tangled more and this affects their properties. Kevlar and nylon are examples of polymers.
Physical Properties The characteristics of a substance that can be observed without changing the substance. n Colour n Size n Shape n Density n Freezing and Boiling Points n Odour

Metals and Nonmetals Metals n Located to the left of the periodic table n Shiny, ductile n Good conductors of heat and electricity Non-metals n Located to the right of the periodic table n Dull and brittle n Poor conductors, good insulators

Metal reactions Metals corrode in the presence of water and oxygen. Only Iron rusts! Metal + Oxygen → Metal oxide • Al + O 2 → Al 2 O 3 Why is Al used for window frames? Metal + Water → Metal hydroxide + Hydrogen • Al + H 2 O → Al(OH)3 + H 2

H. M. S. Sheffield

Metal reactions Metal + Acid → Metal salt + Hydrogen • Mg + HCl → Mg. Cl 2 + H 2 Design an experiment to compare how the rate of a reaction changes with increasing concentration of acid. You can change the concentration of acid by diluting it with water. When I have seen your method and results table you can perform and write up the experiment.

Protecting metals Paint Oil Sacrificial (galvanising) • Zn on Fe Alloys
- Slides: 36