Atoms and Molecules Atoms Atoms are basic building

Atoms and Molecules

Atoms • Atoms are basic building blocks of matter that make up everyday objects.
Atoms • Protons • Neutrons • Electrons
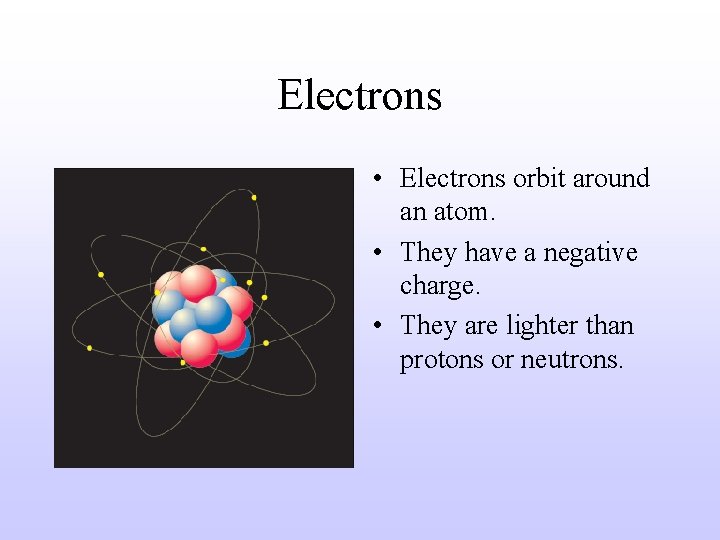
Electrons • Electrons orbit around an atom. • They have a negative charge. • They are lighter than protons or neutrons.
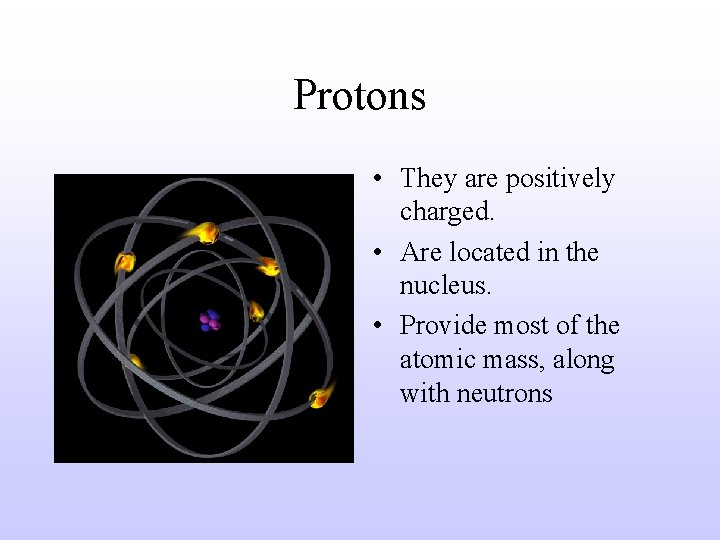
Protons • They are positively charged. • Are located in the nucleus. • Provide most of the atomic mass, along with neutrons
Neutrons • Neutrons are neither positive nor negative. • Also in the nucleus
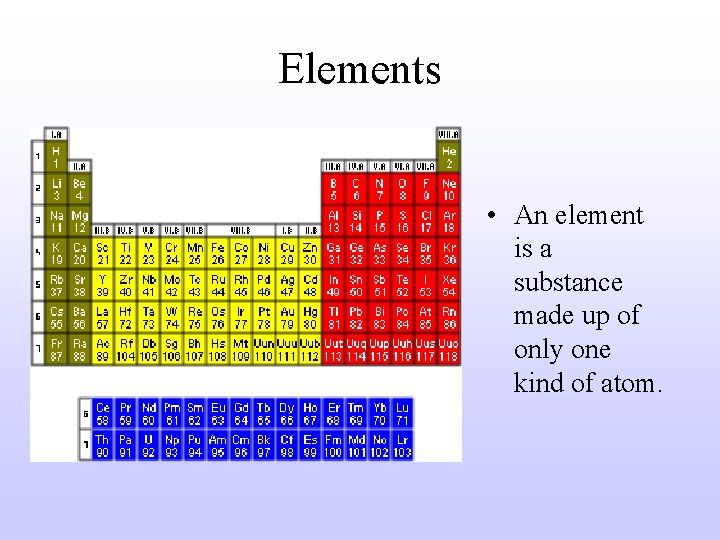
Elements • An element is a substance made up of only one kind of atom.
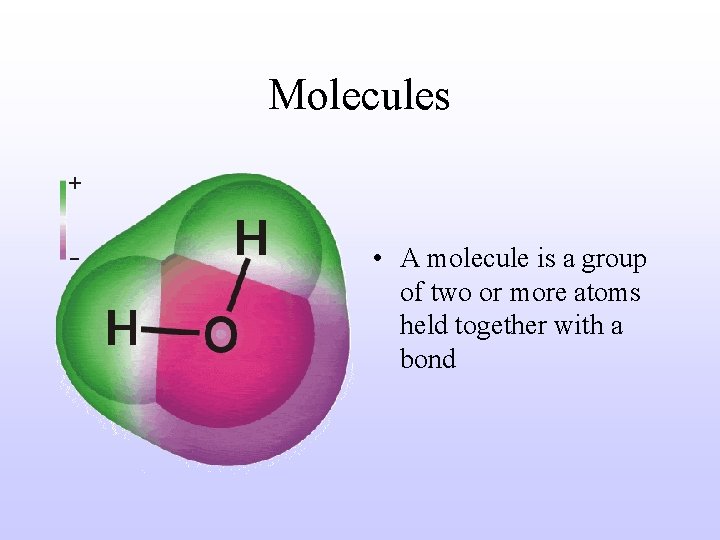
Molecules • A molecule is a group of two or more atoms held together with a bond
Compounds • A compound is two or more elements combined together.
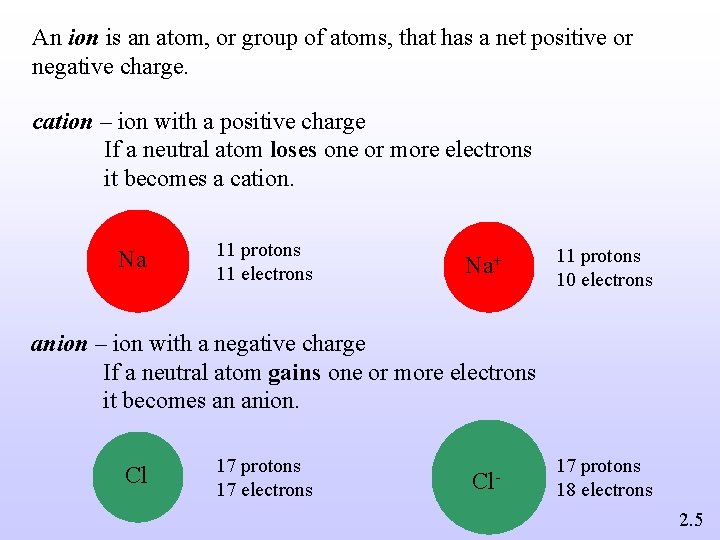
An ion is an atom, or group of atoms, that has a net positive or negative charge. cation – ion with a positive charge If a neutral atom loses one or more electrons it becomes a cation. Na 11 protons 11 electrons Na+ 11 protons 10 electrons anion – ion with a negative charge If a neutral atom gains one or more electrons it becomes an anion. Cl 17 protons 17 electrons Cl- 17 protons 18 electrons 2. 5

A monatomic ion contains only one atom Na+, Cl-, Ca 2+, O 2 -, Al 3+, N 3 - A polyatomic ion contains more than one atom OH-, CN-, NH 4+, NO 3 - 2. 5

Ions • Ions are written with their charge included • If Aluminum loses three electrons, it will be: Al+3 • If Chromium gains an electron, it will be: Cr -1
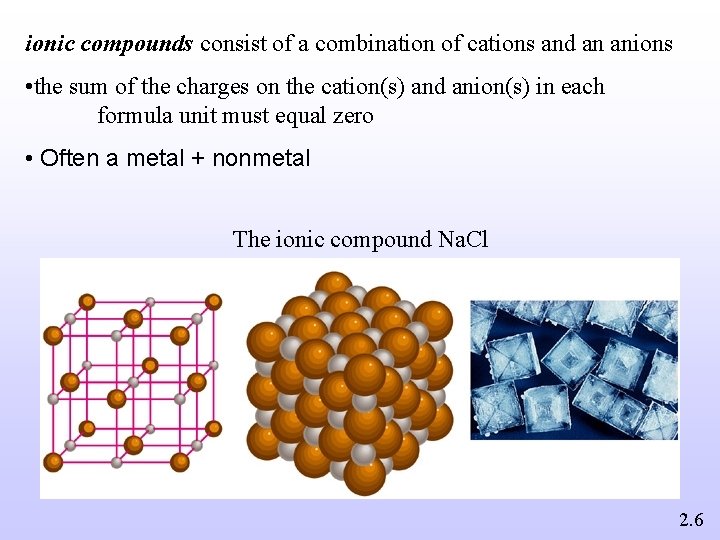
ionic compounds consist of a combination of cations and an anions • the sum of the charges on the cation(s) and anion(s) in each formula unit must equal zero • Often a metal + nonmetal The ionic compound Na. Cl 2. 6
• Molecular compounds – nonmetals or nonmetals + metalloids – common names • H 2 O, NH 3, CH 4, C 60 – element further left in periodic table is 1 st – element closest to bottom of group is 1 st

Why do ionic bonds lose electrons? • Electrons exist in “shells” around the nucleus • Every atom wants their outermost shell to be full • Atoms will gain or lose electrons to make this possible – Examples: Sodium, Potassium, Chlorine

Full Shells • The desire for a full outer shell, or “valence shell” leads atoms to bond with each other in various ways

Chemical Bonds • Chemical Bonds are the attractive force that holds atoms or ions together - 3 types ionic, covalent, metallic

Ionic Bonds - Bond formed by the attraction between oppositely charged ions - oppositely charged ions attract each other and form an ionic bond ex. Na+ + Cl- = Na. Cl
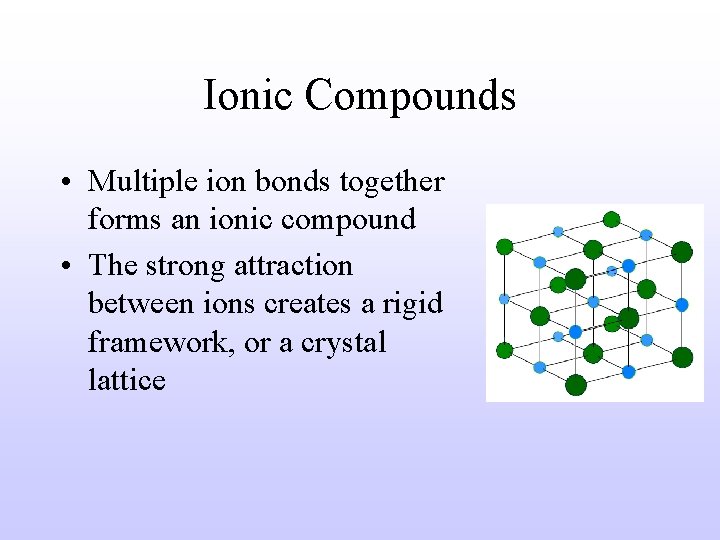
Ionic Compounds • Multiple ion bonds together forms an ionic compound • The strong attraction between ions creates a rigid framework, or a crystal lattice
Covalent Bonds - Chemical bond in which two atoms share a pair of valence electrons - Can be a single, double, or triple bond - Always formed between nonmetals
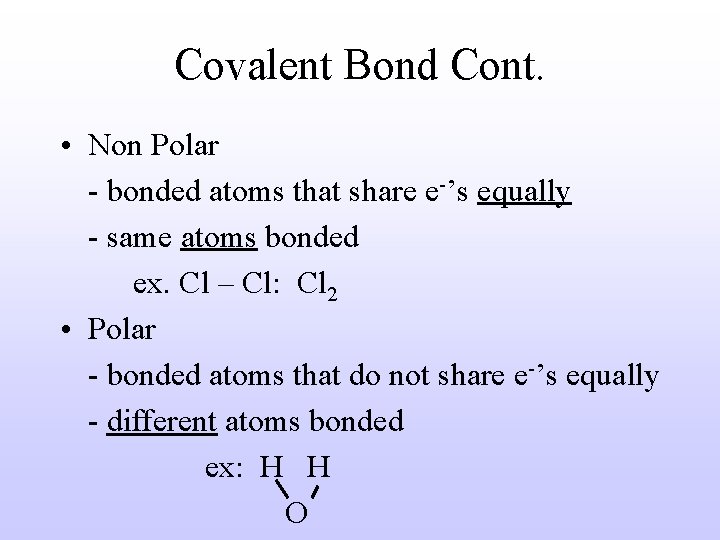
Covalent Bond Cont. • Non Polar - bonded atoms that share e-’s equally - same atoms bonded ex. Cl – Cl: Cl 2 • Polar - bonded atoms that do not share e-’s equally - different atoms bonded ex: H H O

Brief Review Interlude • Electron shells: • Atoms have “shells” of electrons spinning around them • These electrons can exist only in certain numbers in each shell
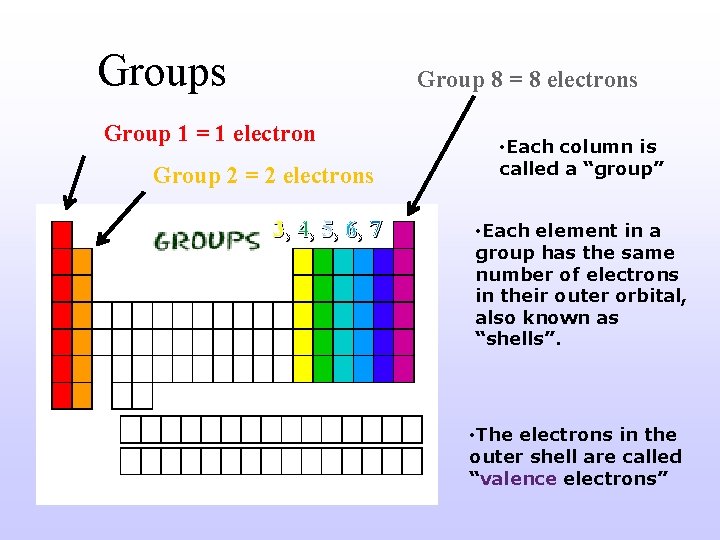
Groups Group 8 = 8 electrons Group 1 = 1 electron Group 2 = 2 electrons 3, 4, 5, 6, 7 • Each column is called a “group” • Each element in a group has the same number of electrons in their outer orbital, also known as “shells”. • The electrons in the outer shell are called “valence electrons”
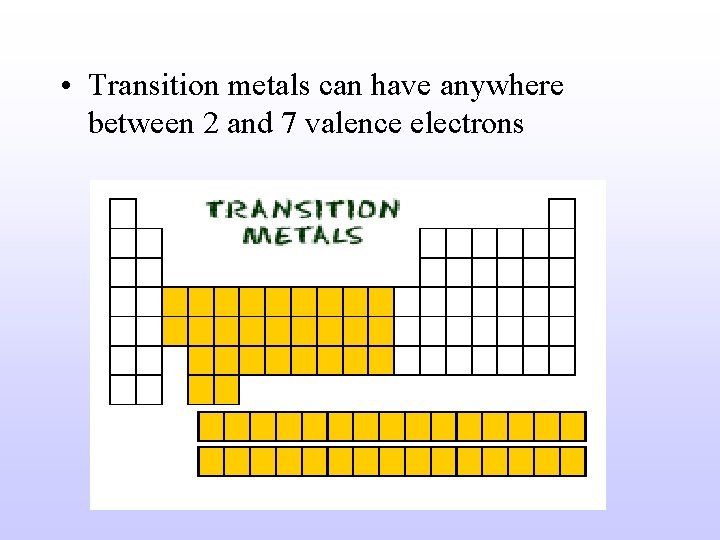
• Transition metals can have anywhere between 2 and 7 valence electrons

Full Shells • The desire for a full outer shell, or “valence shell” leads atoms to bond with each other in various ways

So? • Bellwork: Why are electrons the most important part of a chemical reaction?

Activity • Figure out the valence electrons of your atom(s). Write that number down. • Also: Look in your notes/research. Does it say if your element is more likely to be ionic or covalent?

- Slides: 28