Atoms and Minerals Magnet and Iron and slide



























- Slides: 45

Atoms and Minerals Magnet and Iron and slide Quartz Si. O 2 common mineral

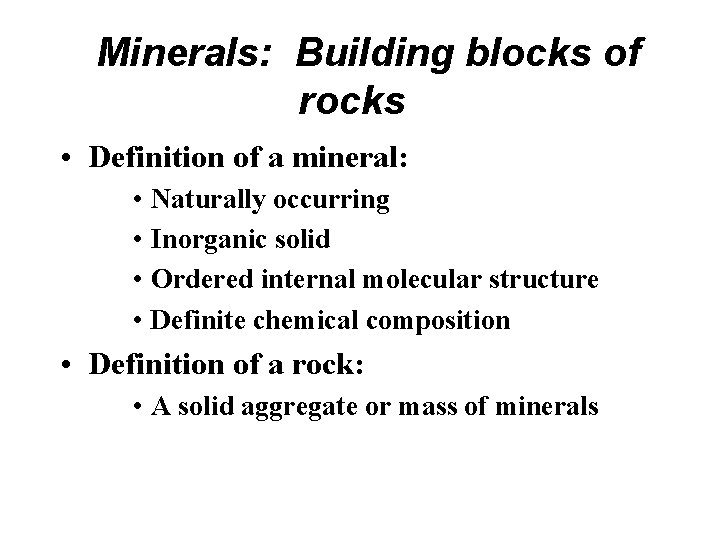
Minerals: Building blocks of rocks • Definition of a mineral: • Naturally occurring • Inorganic solid • Ordered internal molecular structure • Definite chemical composition • Definition of a rock: • A solid aggregate or mass of minerals

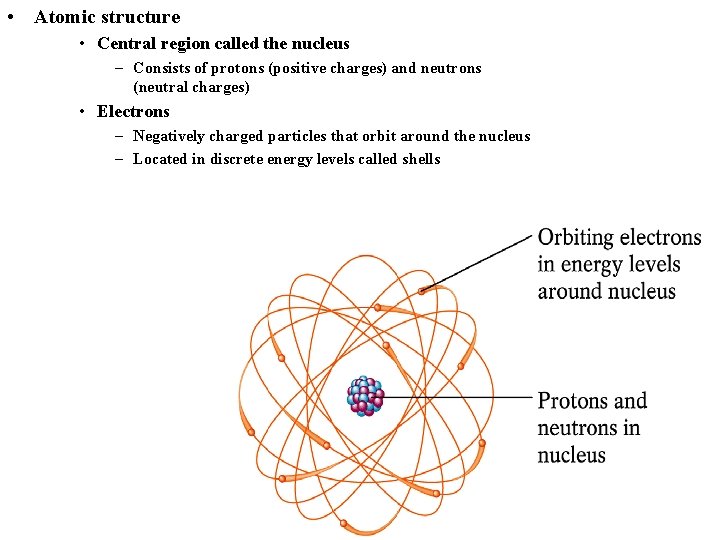
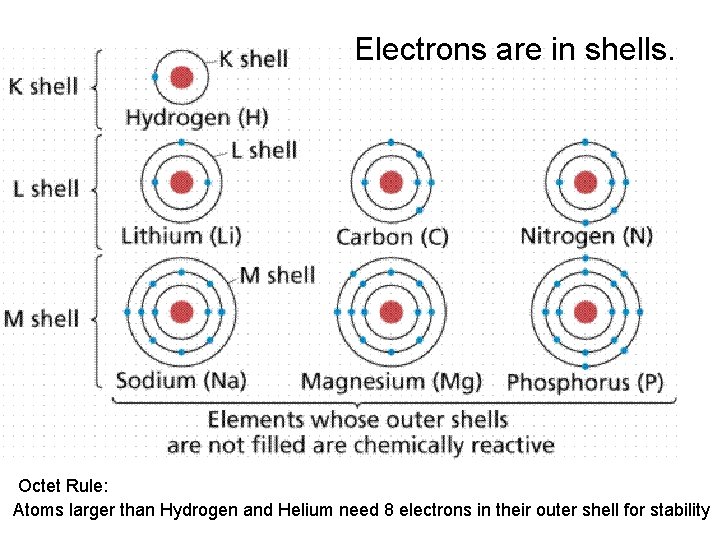
• Atomic structure • Central region called the nucleus – Consists of protons (positive charges) and neutrons (neutral charges) • Electrons – Negatively charged particles that orbit around the nucleus – Located in discrete energy levels called shells

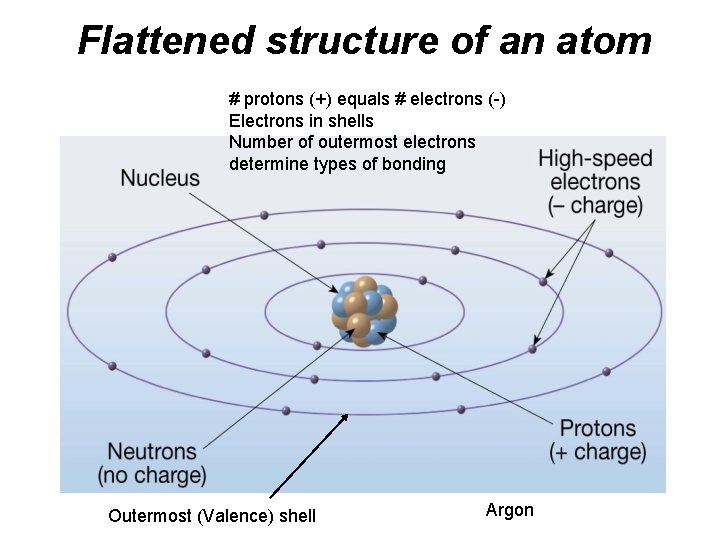
Flattened structure of an atom # protons (+) equals # electrons (-) Electrons in shells Number of outermost electrons determine types of bonding Outermost (Valence) shell Argon

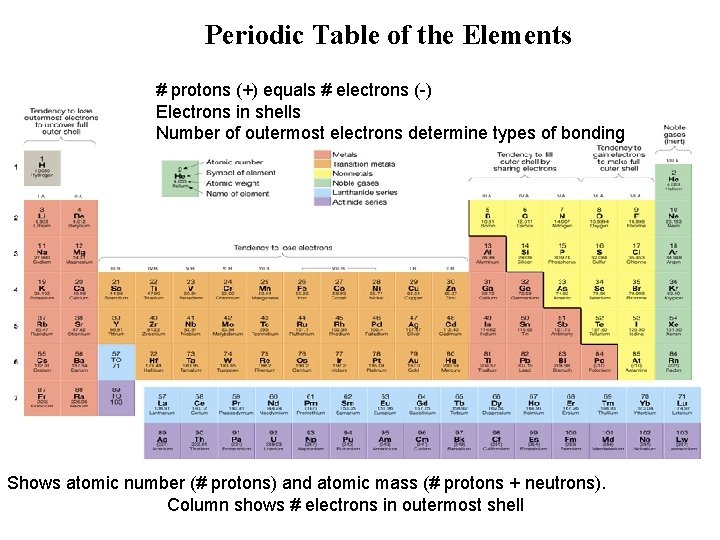
Some definitions: • Atomic number: number of protons in the nucleus • Atomic Mass: total mass of protons and neutrons within an atom’s nucleus • We can see these on a Periodic Table

Periodic Table of the Elements # protons (+) equals # electrons (-) Electrons in shells Number of outermost electrons determine types of bonding Shows atomic number (# protons) and atomic mass (# protons + neutrons). Column shows # electrons in outermost shell

Electrons are in shells. Octet Rule: Atoms larger than Hydrogen and Helium need 8 electrons in their outer shell for stability

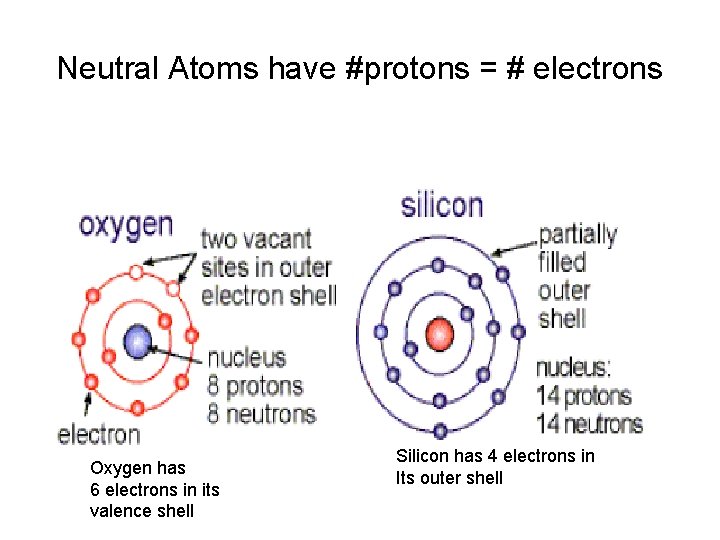
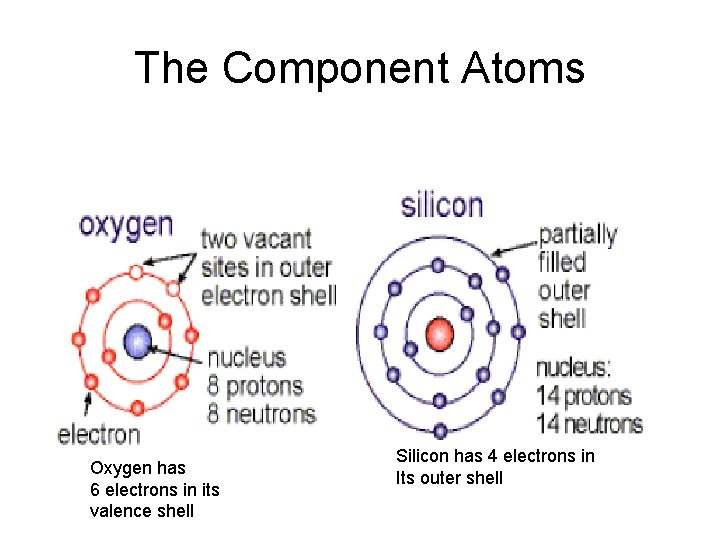
Neutral Atoms have #protons = # electrons Oxygen has 6 electrons in its valence shell Silicon has 4 electrons in Its outer shell

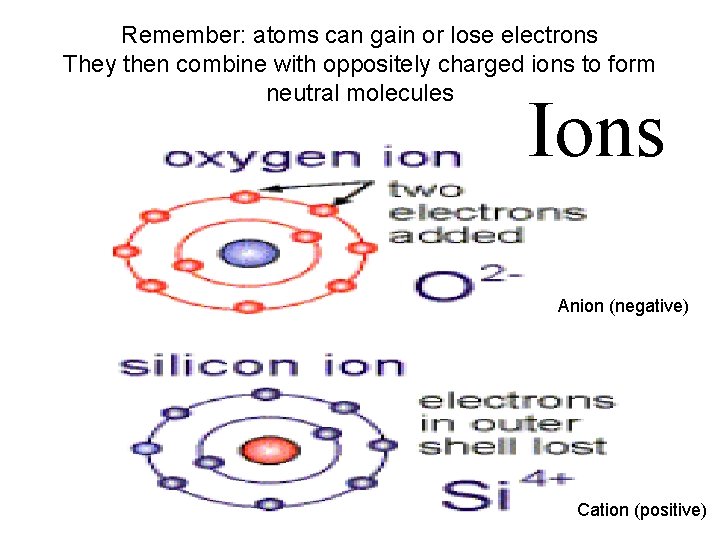
To satisfy the octet rule atoms can gain or lose electrons In that state they are called IONS They can combine with oppositely charged ions to form neutral molecules Oxygen, normally 6 valence electrons, wants 2 extra Ions Silicon, normally 4 valence electrons, would like to be rid of, or share, 4

Chemical Bonding 1: Ionic • Chemical bonding • Formation of a compound by combining two or more atoms • Ionic bonding • Atoms gain or lose outermost (valence) electrons to form ions • Ionic compounds consist of an orderly arrangement of oppositely charged ions • Usually Columns I (alkali metals e. g. Na) and VII (halogens e. g. Cl)

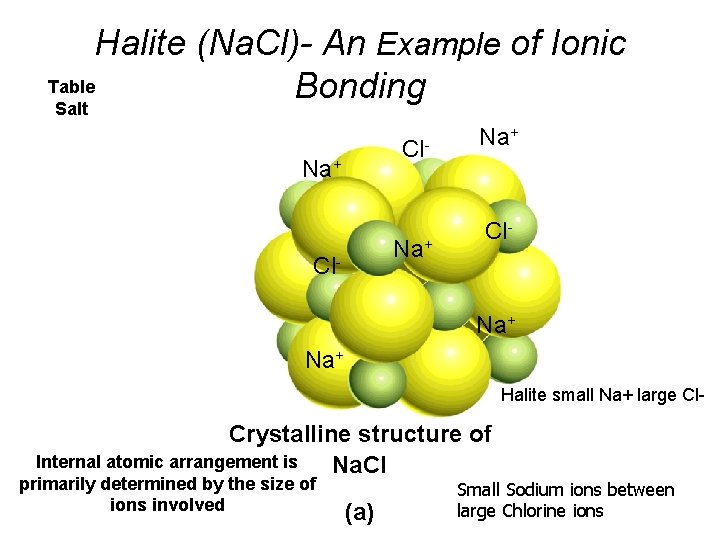
Halite (Na. Cl)- An Example of Ionic Table Bonding Salt Cl- Na+ Na+ Halite small Na+ large Cl- Crystalline structure of Internal atomic arrangement is Na. Cl primarily determined by the size of ions involved (a) Small Sodium ions between large Chlorine ions

Covalent bonding – sharing of valence electrons Sharing Electrons in Outermost Shell Cl 2 Chlorine gas

Covalent Bonds in Water H 2 O Water is polar

Other Bond Types • Metallic bonding – Valence electrons are free to migrate among atoms – Weaker and less common than ionic or covalent bonds • Intermolecular bonding – Hydrogen bonds- charged regions in water attract – Van der Waals bonds- electrons momentarily grouped on same side of nucleus

Isotopes • Isotopes and radioactive decay • Atomic mass is the total mass of neutrons plus protons in an atom • An isotope is an atom that exhibits variation in its atomic mass, i. e. different numbers of neutrons • Some isotopes have unstable nuclei that emit particles and energy in a process known as radioactive decay. • 12 C 13 C stable 14 C radioactive

Structure of minerals • Polymorphs • Two or more minerals with the same chemical composition but different crystalline structures • Diamond and graphite (both carbon) are good examples of polymorphs » The transformation of one polymorph to another is called a phase change » Example: Graphite in a High Pressure Cell Makes Diamond • Some polymorphs make good PT indicators

Diamond and graphite – polymorphs of carbon

Physical properties of minerals • Crystal Form • External expression of the orderly internal arrangement of atoms The mineral garnet often exhibits good crystal form

Physical properties of minerals • Luster • Appearance of a mineral in reflected light • Two basic categories – Metallic – Nonmetallic • Terms are used to further describe nonmetallic luster are vitreous (glassy), pearly, silky, earthy (like dirt), adamantine (greasy)

Galena Pb. S displays metallic luster Valuable ore of Lead

Physical properties of minerals • Color • Generally an unreliable diagnostic property to use for mineral identification • Often highly variable for a given mineral due to slight changes in mineral chemistry • Exotic colorations of some minerals produce gemstones • But we use it anyway Quartz (Si. O 2) exhibits a variety of colors

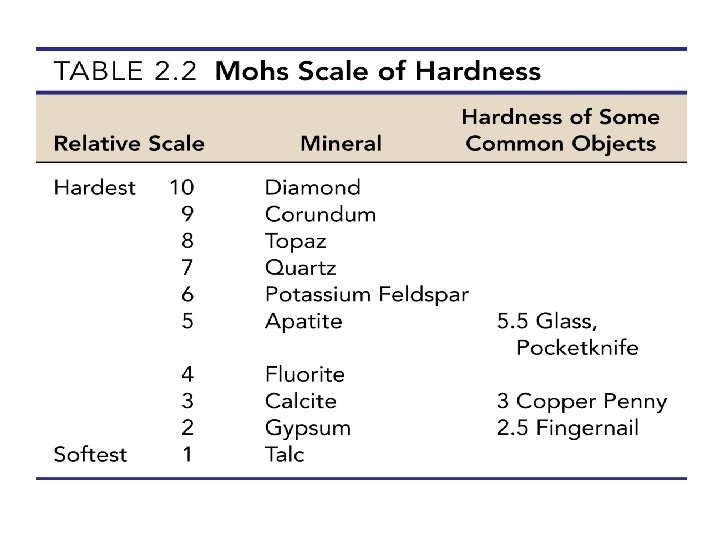
Physical properties of minerals • Streak • Color of a mineral in its powdered form • Helpful in distinguishing different minerals with similar composition • Hardness • Resistance of a mineral to abrasion or scratching • All minerals are compared to a standard scale called the Mohs scale of hardness


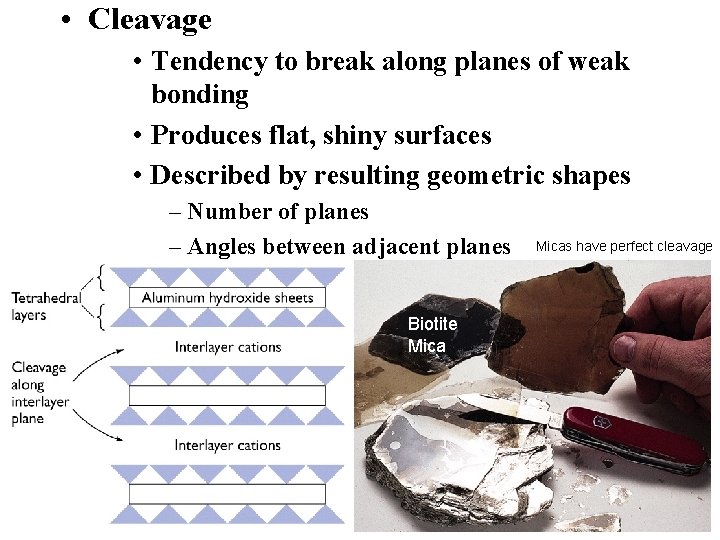
• Cleavage • Tendency to break along planes of weak bonding • Produces flat, shiny surfaces • Described by resulting geometric shapes – Number of planes – Angles between adjacent planes Biotite Micas have perfect cleavage

Three directions of perfect cleavage – fluorite, halite, and calcite Each Cleavage Plane is paired

Physical properties of minerals • Fracture • Absence of cleavage when a mineral is broken. Shown: conchoidal fracture in Quartz • Specific Gravity • Ratio of the weight of a mineral to the weight of an equal volume of water • Average value is approximately 2. 7 • Simply hefting a mineral works too.

Physical properties of minerals • Other properties • Magnetism • Reaction to hydrochloric acid • Malleability • Double refraction • Taste • Smell • Elasticity

Is it calcite or dolomite?

Classification of Minerals • Nearly 4000 minerals have been identified on Earth (We discuss a few) • Rock-forming minerals • Common minerals that make up most of the rocks of Earth’s crust • Only a few dozen members • Composed mainly of the 8 elements that make up 98% of the continental crust

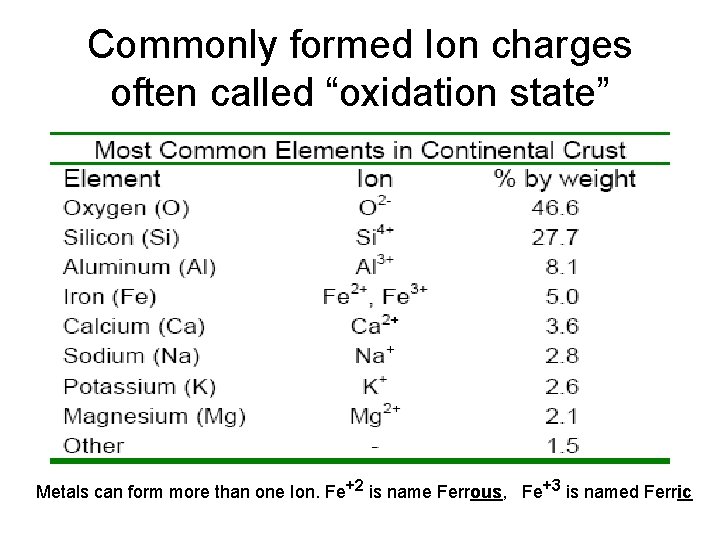
Commonly formed Ion charges often called “oxidation state” Metals can form more than one Ion. Fe+2 is name Ferrous, Fe+3 is named Ferric

Classification of Minerals • Silicates • Most important mineral group – Comprise most of the rock-forming minerals – Very abundant due to large amounts of silicon and oxygen in Earth’s crust • Basic building block is the silicon-oxygen tetrahedron molecule – Four oxygen ions surrounding a much smaller silicon ion

The Component Atoms Oxygen has 6 electrons in its valence shell Silicon has 4 electrons in Its outer shell

Remember: atoms can gain or lose electrons They then combine with oppositely charged ions to form neutral molecules Ions Anion (negative) Cation (positive)

Silicate Molecule The Silicon-Oxygen Tetrahedron 2_25 O 2 - Si 4+ O 2 - O 2 The basis of most rock-forming minerals, charge - 4 O 2 -

Silicate Bonding I • Oxygen O atoms may obtain electrons from Si atoms, producing the Si. O 4 -4 Ion. • The negative charge is balanced by positive metal ions. • This occurs in Olivine, (Fe, Mg)2 Si. O 4, a high temperature Fe-Mg silicate. Forms of this mineral are stable 100’s of kilometers below Earth’s surface. • Sort of Ionic Bond

Example OLIVINE Positive ion Fe and Mg Si. O 4 -4 Ion Tetrahedron facing down Tetrahedron facing up Independent tetrahedra

Silicate Bonding II • Alternately, the oxygen atoms may complete their outer electron shells by sharing electrons with two Silicon atoms in nearby silicon tetrahedra. • A sort of covalent bond

A Pyroxene Single chains weakly paired

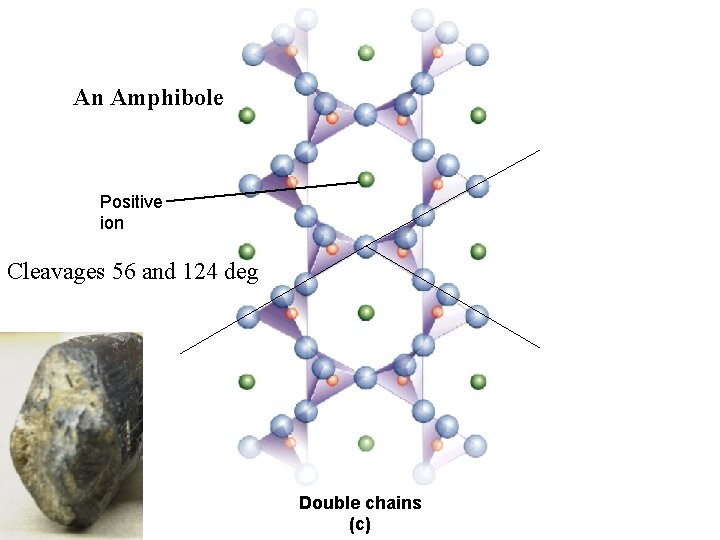
2_26 c An Amphibole Positive ion Cleavages 56 and 124 deg Double chains (c)

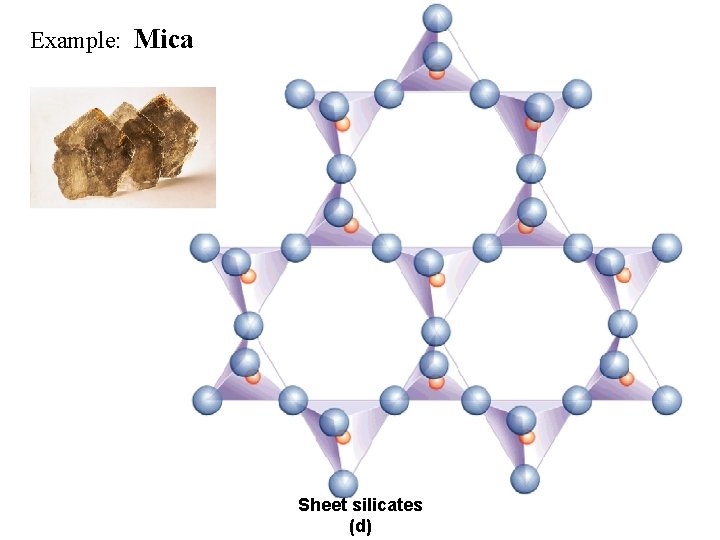
Example: Mica Sheet silicates (d)

Clay Minerals (at high magnification) note sheet structure Very small crystals Kaolinite (hand specimen) Clays are also Sheet Silicates, just as Micas are Vietnam Anecdote

Example: Quartz Si. O 2 2_26 e Framework silicates (e) (3 -D, also the Feldspars)

Classification of Minerals • Common Silicate minerals • Feldspar Group – Most common mineral group – two directions of perfect cleavage at 90 degrees – In Feldspars, some of the Silicon atoms (oxidation state +4) are replaced by Aluminum (oxidation state +3) – Ion is not symmetrical – Pearly Luster A Potassium Feldspar

Feldspars that use Calcium (Ca) or Sodium (Na) metals to balance the Si. O 4 - 4 and Al. O 4 -5 charges are called: Plagioclase feldspar Note the Twinning, seems to have ‘stripes’

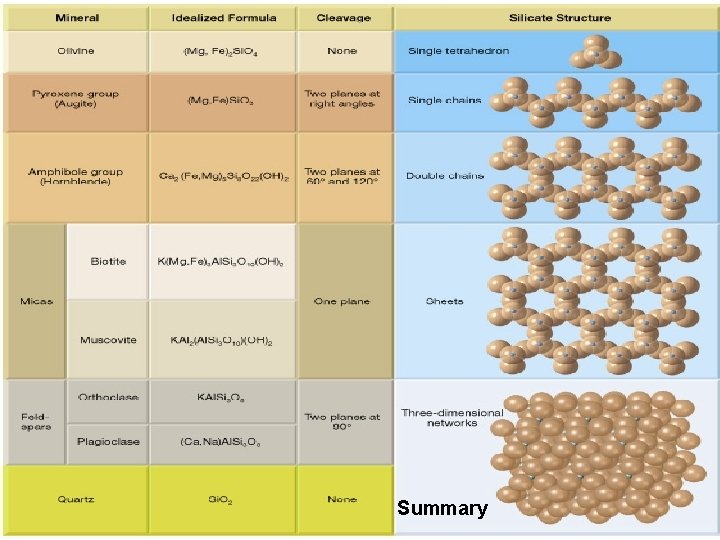
Summary
 What are the basic dance steps in heel and toe polka?
What are the basic dance steps in heel and toe polka? Difference between permanent magnet and temporary magnet
Difference between permanent magnet and temporary magnet Temporary magnet
Temporary magnet Apakah magnet berasal dari kata magnesia
Apakah magnet berasal dari kata magnesia At stp which substance is the best conductor of electricity
At stp which substance is the best conductor of electricity Magnetism test review
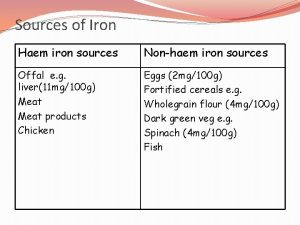
Magnetism test review Mass of iron in an iron tablet
Mass of iron in an iron tablet Iron sharpens iron friendship
Iron sharpens iron friendship Factoring polynomials
Factoring polynomials Function of minerals
Function of minerals Rocks and minerals song
Rocks and minerals song Solid rock
Solid rock Chapter 8 vitamins and minerals
Chapter 8 vitamins and minerals Minor elements
Minor elements Difference between rocks and minerals
Difference between rocks and minerals Concept map rocks and minerals
Concept map rocks and minerals Suzanna socked me sunday poem
Suzanna socked me sunday poem Absorbs water and minerals
Absorbs water and minerals What kind of rock is this
What kind of rock is this Padma mines and minerals corporation
Padma mines and minerals corporation Stores minerals and anchors muscles
Stores minerals and anchors muscles Cumbria minerals and waste local plan
Cumbria minerals and waste local plan Resource that can be replaced
Resource that can be replaced Minerals and fuels
Minerals and fuels Vitamin deficiency diseases chart
Vitamin deficiency diseases chart Minerals and fuels
Minerals and fuels Importance of mineralogy
Importance of mineralogy Classification of minerals
Classification of minerals Rock type
Rock type Biaxial indicatrix
Biaxial indicatrix Are vitamins and minerals macromolecules
Are vitamins and minerals macromolecules Streak of minerals
Streak of minerals Conflict minerals
Conflict minerals Storage of minerals
Storage of minerals Primary vs secondary minerals
Primary vs secondary minerals Minerals vs rocks
Minerals vs rocks Rocks are aggregates of minerals
Rocks are aggregates of minerals Cleavage of minerals
Cleavage of minerals Properties of minerals
Properties of minerals Duresa dels minerals
Duresa dels minerals Luster
Luster Fracture minerals
Fracture minerals Characteristics of a mineral
Characteristics of a mineral Minerals can be identified by
Minerals can be identified by Nearly 4000 minerals have been named
Nearly 4000 minerals have been named Pumice rock type
Pumice rock type