Atoms and Macromolecules Key Questions 1 What three











- Slides: 30

Atoms and Macromolecules

Key Questions 1. What three subatomic particles make up atoms? 2. In what ways does a compound differ from its component elements? 3. What elements does carbon bond with to make up life’s molecules? 4. What are the functions of each of the four groups of macromolecules?

What are you made of? When you breathe, eat, or drink, your body uses the substances in air, food, and water to carry out chemical reactions that keep you alive.

What is the basic unit of matter? ● The atom!

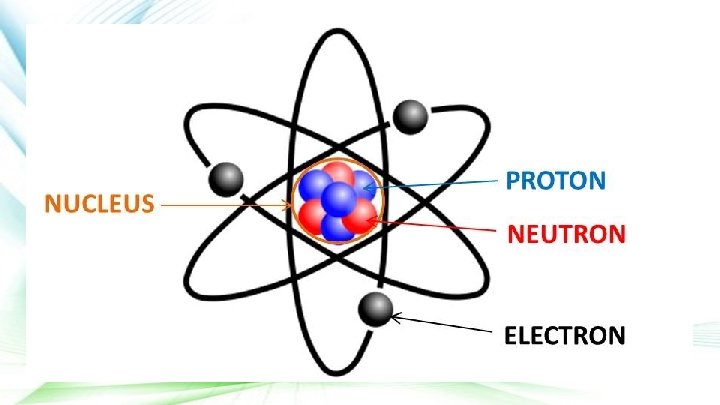
Atoms ● Atom is the Greek word meaning “unable to cut” ○ They are the smallest part of an element that maintains the properties of that element

With your desk partner(s) discuss: ○ What are the components of an atom? ○ Include where they are found and their charge.


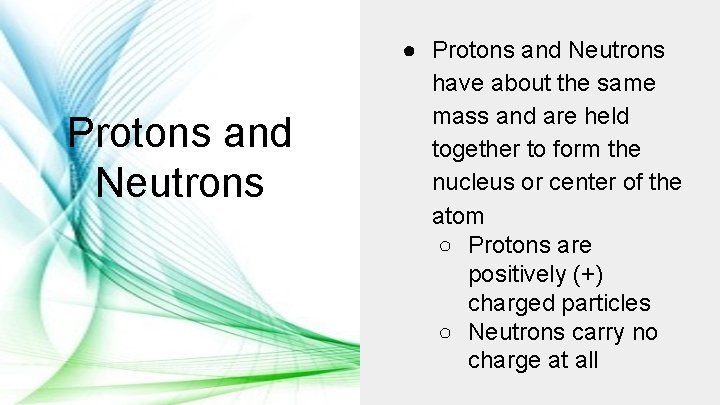
Protons and Neutrons ● Protons and Neutrons have about the same mass and are held together to form the nucleus or center of the atom ○ Protons are positively (+) charged particles ○ Neutrons carry no charge at all

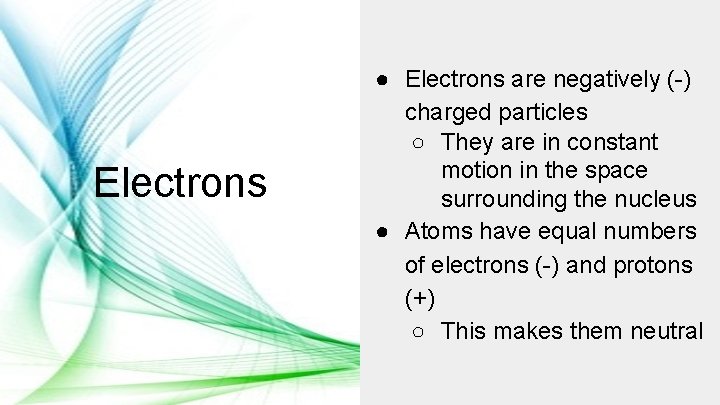
Electrons ● Electrons are negatively (-) charged particles ○ They are in constant motion in the space surrounding the nucleus ● Atoms have equal numbers of electrons (-) and protons (+) ○ This makes them neutral

An element is a pure substance that consists entirely of one type of atom.

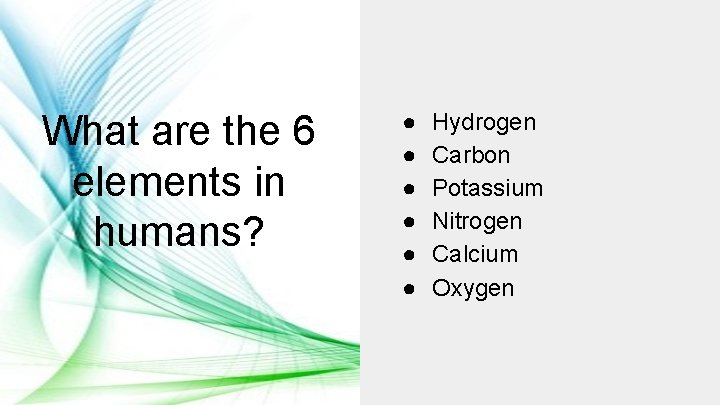
What are the 6 elements in humans? ● ● ● Hydrogen Carbon Potassium Nitrogen Calcium Oxygen



In nature, most elements are found combined with other elements in compounds. A chemical compound is a substance formed by the combo of two or more elements.

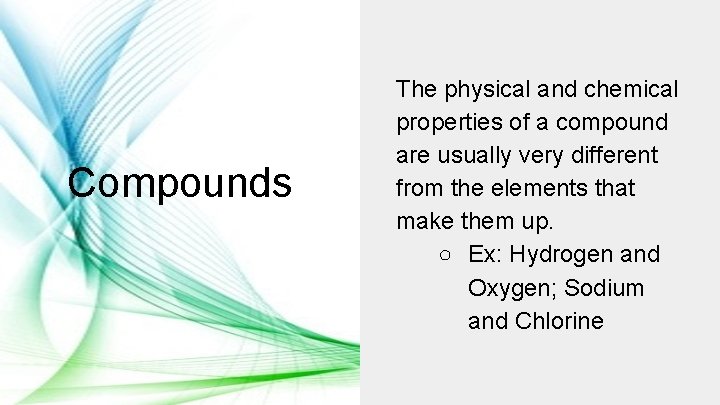
Compounds The physical and chemical properties of a compound are usually very different from the elements that make them up. ○ Ex: Hydrogen and Oxygen; Sodium and Chlorine

Carbon Compounds “Organic Chemistry”

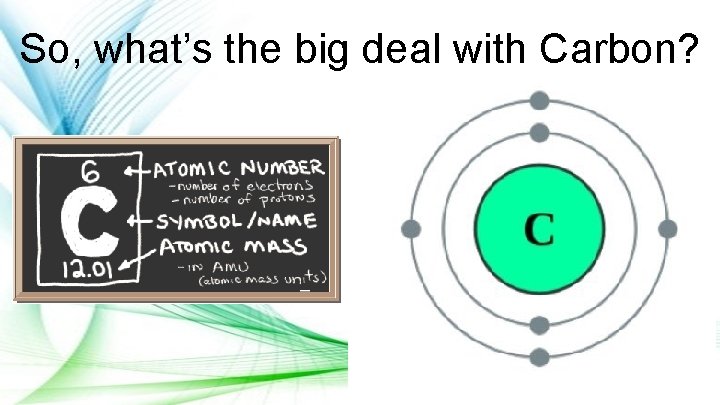
So, what’s the big deal with Carbon?

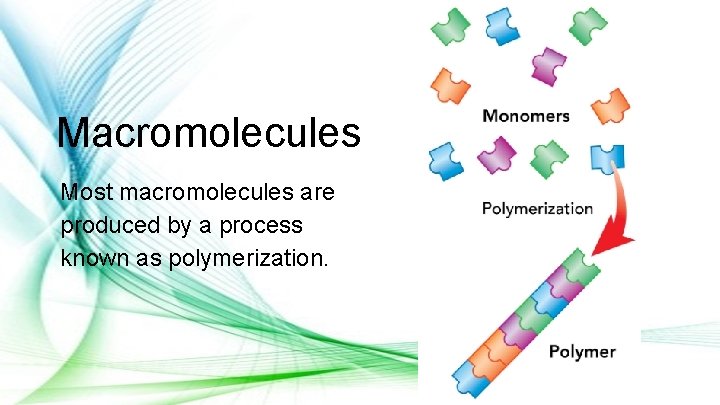
The most important carbon compounds to living things are called Macromolecules.

Macromolecules Most macromolecules are produced by a process known as polymerization.

What are the 4 macromolecules found in living things? 1. 2. 3. 4. Carbohydrates Lipids Proteins Nucleic Acids

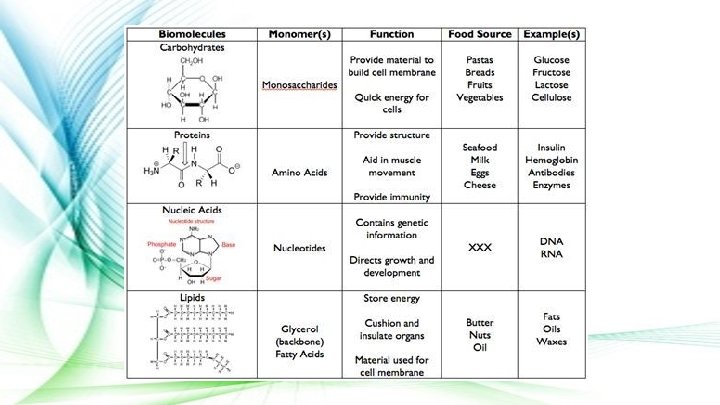
Carbohydrates ● Made up of Carbon, Hydrogen, and Oxygen atoms ● Functions: Organisms use carbohydrates to store and release energy ● Composed of chains of monosaccharides like glucose

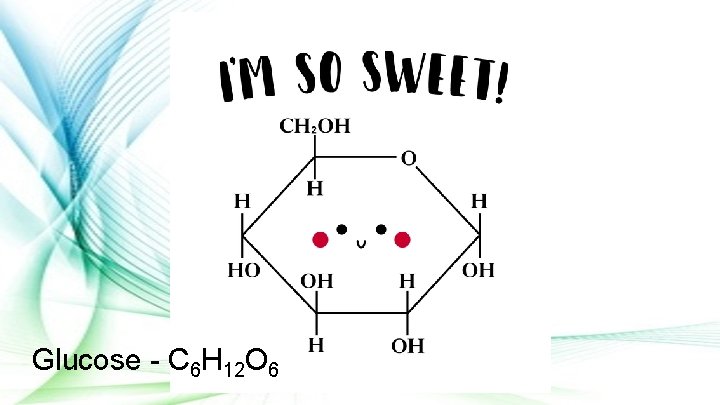
Glucose - C 6 H 12 O 6

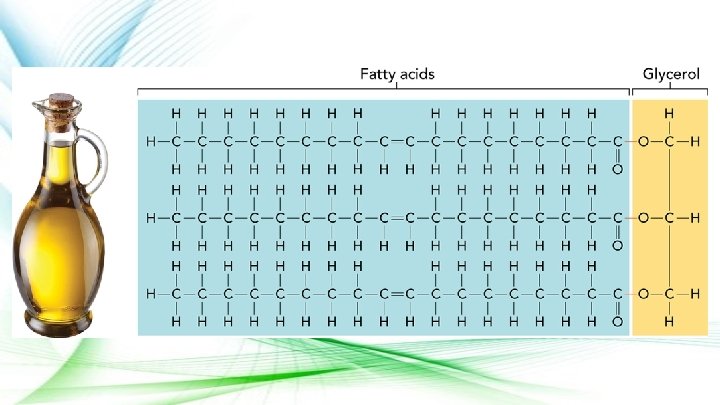
Lipids ● Made up of Carbon and Hydrogen atoms ● Function: Organisms use lipids to store energy, and they form important parts of biological membranes and waterproof coverings ● Usually composed of chains of fatty acids


Proteins ● Made up of Nitrogen, Carbon, Hydrogen, and Oxygen atoms ● Function: Organisms use proteins for structure, support and enzyme production (speeds up chemical reactions) ● Composed of chains of amino acids


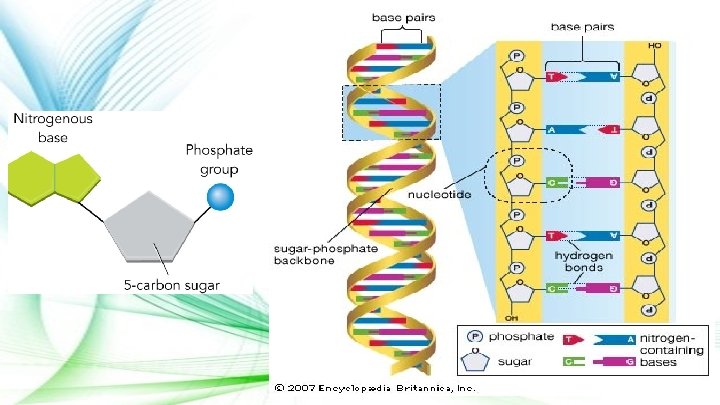
Nucleic Acids ● Found in most cells in the form of DNA & RNA ● Function: provides the instructions for all life processes ● Composed of chains of nucleotides


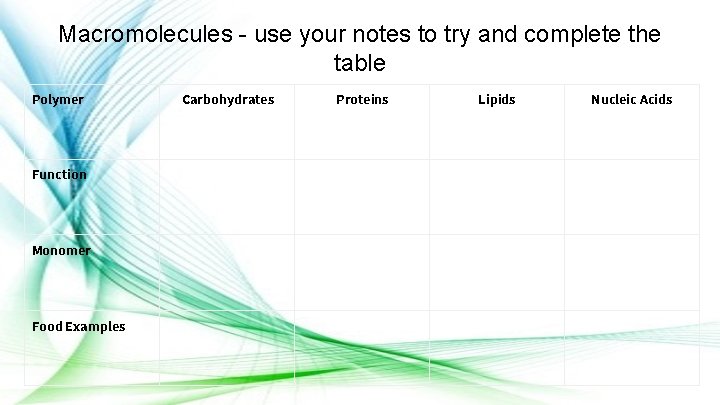
Macromolecules - use your notes to try and complete the table Polymer Function Monomer Food Examples Carbohydrates Proteins Lipids Nucleic Acids
