Atoms and Ions Pg 188 191 Valence Electrons









- Slides: 16

Atoms and Ions Pg 188 - 191

Valence Electrons l Valence Electrons are the outermost electrons found in an atom. l These electrons determine the type of ion an atom will form, and how an atom can bond to other atoms.

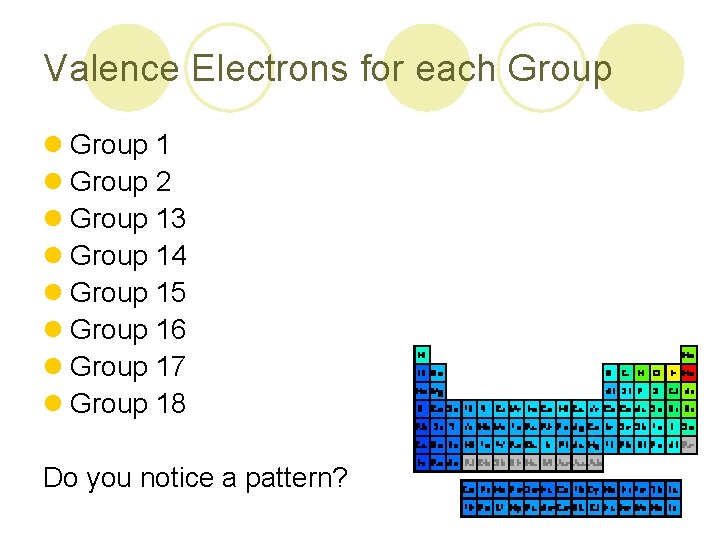
Valence Electrons for each Group l Group 1 l Group 2 l Group 13 l Group 14 l Group 15 l Group 16 l Group 17 l Group 18 Do you notice a pattern?

Lewis Diagrams (aka Electron Dot Diagrams) l Lewis diagrams show ONLY the valence electrons for an element. l Draw one dot each: on the top, right, bottom, and left of the element symbol, then start doubling up. (12, 3, 6, 9 on a clock) l Draw the Lewis Diagrams for the first 20 Elements on your handout.

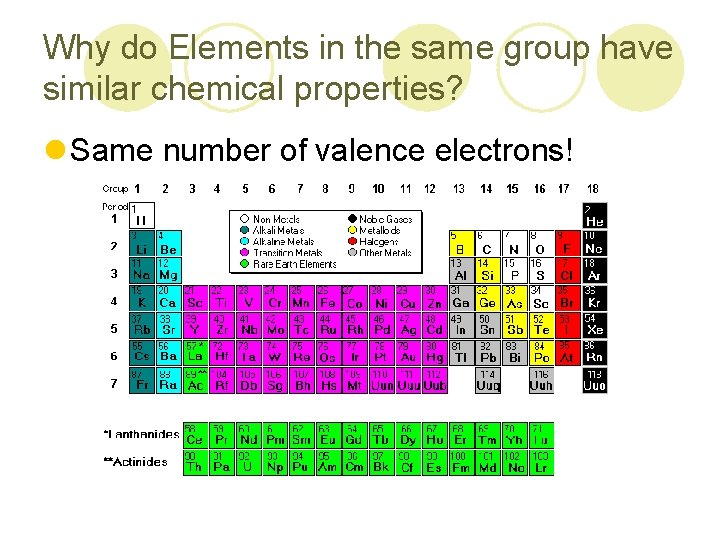
Why do Elements in the same group have similar chemical properties? l Same number of valence electrons!

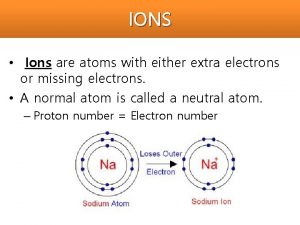
Atoms & Ions l Atoms have equal numbers of protons and electrons and are therefore electrically neutral l Atoms are not stable unless their outer electron orbits are full l This is achieved by either gaining, losing, or sharing electrons.

Atoms and Ions l When an atom gains or loses one or more electrons, it becomes an ion. It is now electrically charged, but stable. l The ionic charge is the sum of the ion’s positive and negative charges.

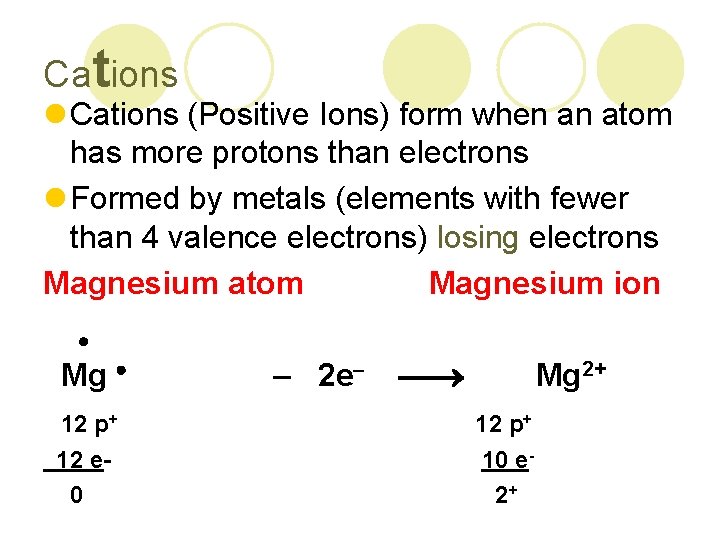
Cations l Cations (Positive Ions) form when an atom has more protons than electrons l Formed by metals (elements with fewer than 4 valence electrons) losing electrons Magnesium atom Magnesium ion Mg – 2 e Mg 2+ 12 p+ 12 e 0 10 e 2+

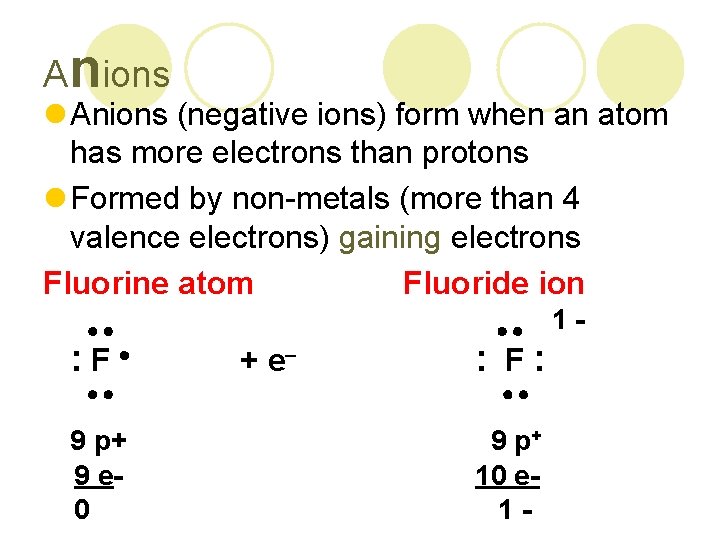
Anions l Anions (negative ions) form when an atom has more electrons than protons l Formed by non-metals (more than 4 valence electrons) gaining electrons Fluorine atom Fluoride ion : F 9 p+ 9 e 0 + e : F: 9 p+ 10 e 1 - 1 -

“Cats have Paws” l Here’s another little trick to help you remember what anions and cations are: ¡Cations are Positive ¡Cats have Paws

Ion Joke Two hydrogen atoms bumped into each other recently. One said: "Why do you look so sad? " The other responded: "I lost an electron. " Concerned, One asked "Are you sure? " The other replied "I'm positive. "


And now for something completely different…. l On your electron diagrams, write the symbol for the ion formed by each of the first 20 elements l The charge on each ion is called the valence number l Let’s compare….

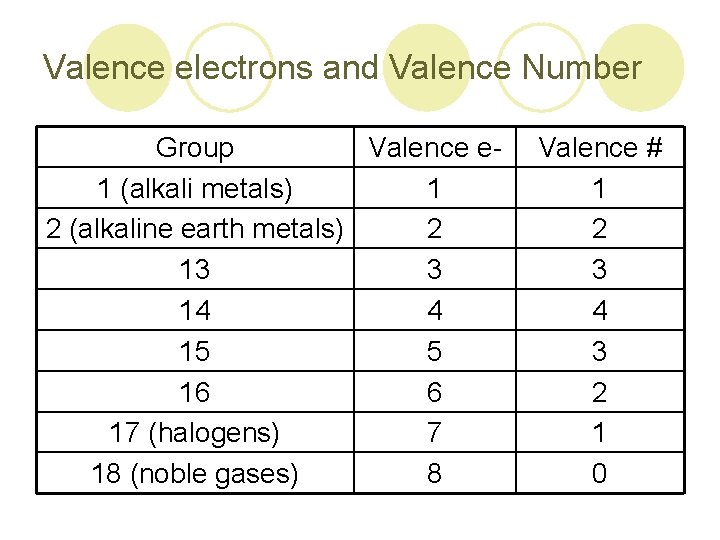
Valence electrons and Valence Number Group Valence e 1 (alkali metals) 1 2 (alkaline earth metals) 2 13 3 14 4 15 5 16 6 17 (halogens) 7 18 (noble gases) 8 Valence # 1 2 3 4 3 2 1 0

How Cool is That? ? !!!

You’ll get a charge out of this…. Page 191 #1 - 6, 8, 9 & Try This #2: Ions and the Periodic Table
 Atoms with 4 valence electrons
Atoms with 4 valence electrons Label an atom
Label an atom How do chemists model the valence electrons of metal atoms?
How do chemists model the valence electrons of metal atoms? How do chemists model the valence electrons of metal atoms?
How do chemists model the valence electrons of metal atoms? Carbon trichloride
Carbon trichloride Atoms molecules and ions
Atoms molecules and ions Atoms molecules and ions
Atoms molecules and ions Atoms molecules and ions
Atoms molecules and ions Atoms molecules and ions
Atoms molecules and ions Atoms ions and molecules
Atoms ions and molecules Atoms ions and molecules
Atoms ions and molecules States that atoms ions and molecules must collide to react
States that atoms ions and molecules must collide to react Chapter 2 atoms molecules and ions
Chapter 2 atoms molecules and ions Life on the edge valence and core electrons answer key
Life on the edge valence and core electrons answer key 1s 22 s22 p63 s23 p64 s2 3d 10
1s 22 s22 p63 s23 p64 s2 3d 10 Electrons in atoms section 1 light and quantized energy
Electrons in atoms section 1 light and quantized energy Electrons in atoms section 1 light and quantized energy
Electrons in atoms section 1 light and quantized energy