Atoms and Atomic Theory Overview Atomic Structure protons


































- Slides: 43

Atoms and Atomic Theory Overview • Atomic Structure (protons, electrons, neutrons) • Isotopes • Electron shells, energy levels • Periodic table

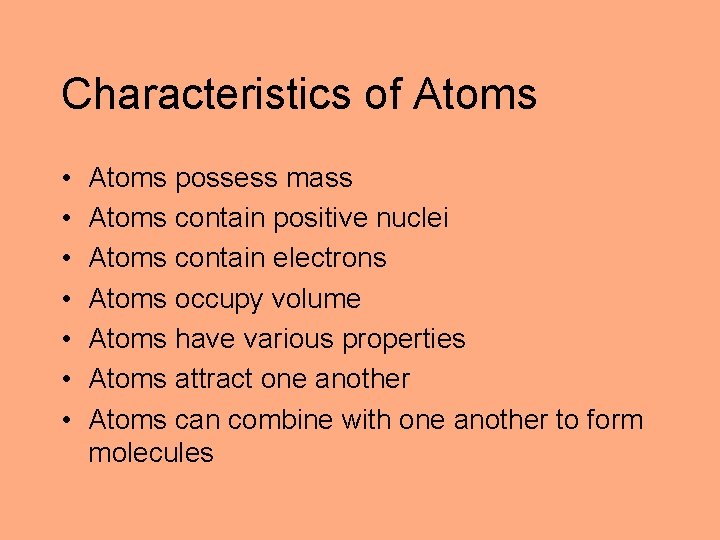
Characteristics of Atoms • • Atoms possess mass Atoms contain positive nuclei Atoms contain electrons Atoms occupy volume Atoms have various properties Atoms attract one another Atoms can combine with one another to form molecules

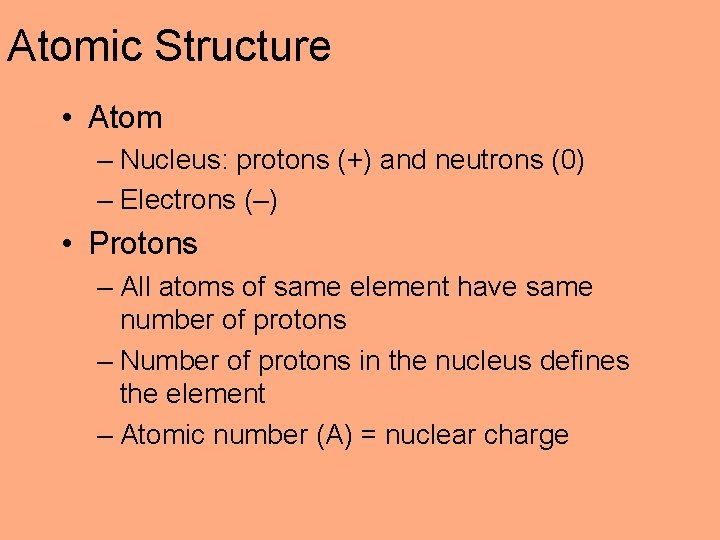
Atomic Structure • Atom – Nucleus: protons (+) and neutrons (0) – Electrons (–) • Protons – All atoms of same element have same number of protons – Number of protons in the nucleus defines the element – Atomic number (A) = nuclear charge

Atomic Number Symbol and atomic number signify same thing.

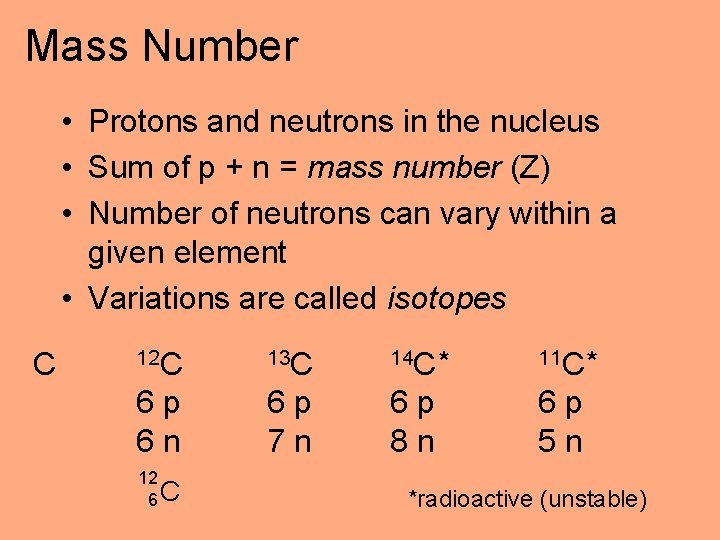
Mass Number • Protons and neutrons in the nucleus • Sum of p + n = mass number (Z) • Number of neutrons can vary within a given element • Variations are called isotopes C 12 C 13 C 14 C * 11 C * 6 p 6 n 6 p 7 n 6 p 8 n 6 p 5 n 12 6 C *radioactive (unstable)

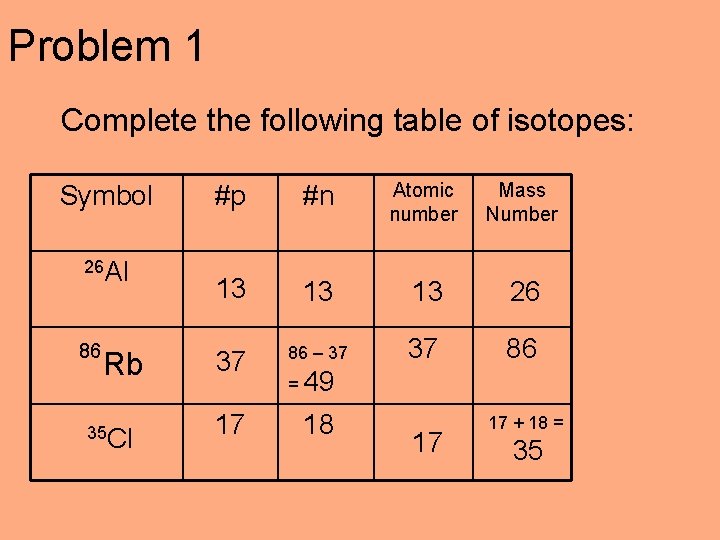
Problem 1 Complete the following table of isotopes: Symbol #p #n Atomic number Mass number 37 86 26 Al 17 18

Problem 1 Complete the following table of isotopes: Symbol 26 Al 86 Rb 35 Cl #p #n Atomic number Mass Number 13 13 13 26 37 86 – 37 37 86 17 18 = 49 17 17 + 18 = 35

Atomic Weight • Weighted average of all naturally occurring isotopes • Mass number = n + p, whole number, particular isotope • Atomic weight, decimal, – combination of all isotopes naturally found – experimental number

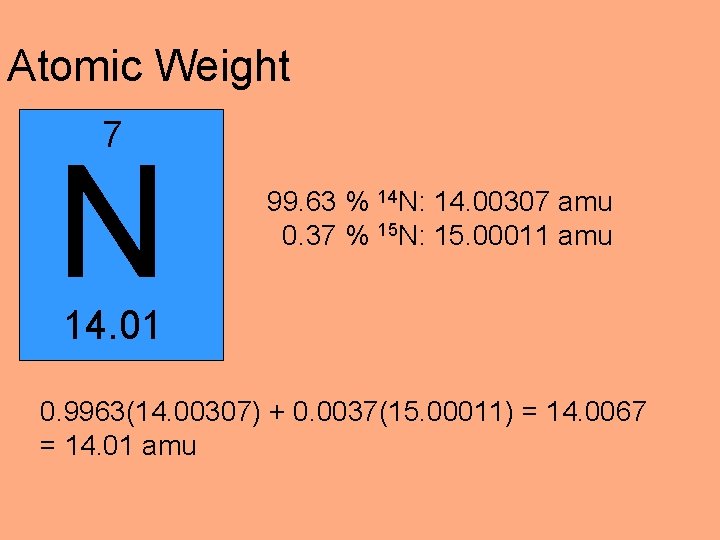
Atomic Weight 7 N 99. 63 % 14 N: 14. 00307 amu 0. 37 % 15 N: 15. 00011 amu 14. 01 0. 9963(14. 00307) + 0. 0037(15. 00011) = 14. 0067 = 14. 01 amu

Problem 2 Magnesium has three naturally occurring isotopes with the following masses and abundances: 23. 99 amu 78. 99 % 24. 99 amu 10. 00 % 25. 98 amu 11. 01 % Calculate the atomic weight of magnesium.

Problem 2 (23. 99 amu)(0. 7899) + (24. 99 amu)(0. 1000) + (25. 98 amu)(0. 1101) = 24. 31 amu correlation to grams

The “Mole” • Chemists use “moles” as a way to count atoms which are so tiny • Industry analogs • Atomic weight (molar mass) connects gram amount to atomic mass units (which is related to proton/neutron mass) • Avogadro’s number: 6. 02 x 1023 • Numerically correlated: 24. 31 amu 24. 31 g/mol Know grams know atoms

Periodic Table • • Electronic structure Bohr Model flawed but functional Electronic energy levels How do scientists (chemists) use models?

Models • Useful to simplify complex or confusing systems • Understand behavior of aspects of universe • Limitations, oversimplification • Atomic models vs. atomic theory

Law versus Theory Discuss each of the following terms (as is typically done in society). Give an example of each. a) Law b) Theory

Scientific Perspective • Law: generalization based upon observation (measurement) to which there are no exceptions – Law of gravity – Gas Laws – Newton’s Laws of Motion • No theoretical framework, empirically based

Scientific Perspective • Theory: model that describes underlying cause of physical behavior (law) – Predictive – Goes beyond laws from which formulated – Testable (experiment) • Examples – Kinetic Molecular Theory – Atomic Theory • How do models fit?

Atomic Theory • Atomic Theory and Quantum Mechanical model of the atom developed through interaction of matter with light (electromagnetic radiation)

Electromagnetic Spectrum

Visible Spectrum

Bohr Model Line spectra Light through a prism continuous spectrum: Ordinary white light

Bohr Model Line spectra Light from gas-discharge tube through a prism line spectrum: H 2 discharge tube

Line Spectra (emission) White light H He Ne

Line Spectra (absorption) Gas-filled tube Light source

Electronic Energy Levels n = electronic energy level n = 1 2 electrons (H, He) n = 2 8 electrons (Li Ne) NOT orbits, energy levels 2 e– 8 e–

Electronic Energy Levels n = main electronic energy level Sublevels: s, p, d, f n = 1, s only: 2 electrons maximum n = 2, s & p: 2 electrons in s, 6 electrons in p n = 3, s, p & d: 2 electrons in s, 6 electrons in p, 10 electrons in d n = 4, s, p, d, f: s-2, p-6. d-10, f has 14 Filling order is unexpected

Electronic Energy Levels Filling order 1 s 2 s 3 s 4 s 5 s 6 s 7 s 2 p 3 p 4 p 5 p 6 p 7 p 3 d 4 d 5 d 6 d 4 f 5 f 5 g

Electronic Energy Levels n = electronic energy level Sublevels: s, p, d, f Periodic table can be “derived” from energy levels n = 3 s and p fill first: 8 electrons (Na Ar) n=4 4 s fills before 3 d (K, Ca) but 3 d fills before 4 p (Sc Zn) 2 e– 4 p (Ga Kr) 8 e–

Periodic Table (p. 293) n=1 n=2 n=3 n=4

Electronic Energy Levels • Inner shell versus outer shell electrons • Inner shell: not involved in formation of chemical bonds • Outer shell: involved in formation of chemical bonds • Outer shell valence electrons

Valence Electrons 1 e- 2 e- 3 e- 4 e- 5 e- 6 e- 7 e- 8 e- n=1 H He n=2 Li Be B C N O F Ne n=3 Na Mg Al Si P S Cl Ar

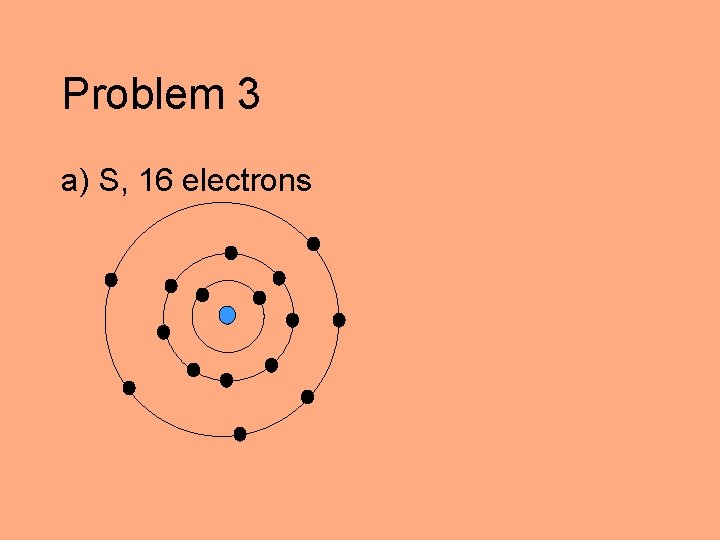
Problem 3 Draw the electron configurations for each of the following elements according to the Bohr model. Indicate which electrons are valence electrons. a) S b) Na c) C

Problem 3 a) S, 16 electrons

Problem 3 a) S, 16 electrons

Problem 3 b) Na, 11 electrons

Problem 3 b) Na, 11 electrons

Problem 3 c) C, 6 electrons

Problem 3 c) C, 6 electrons

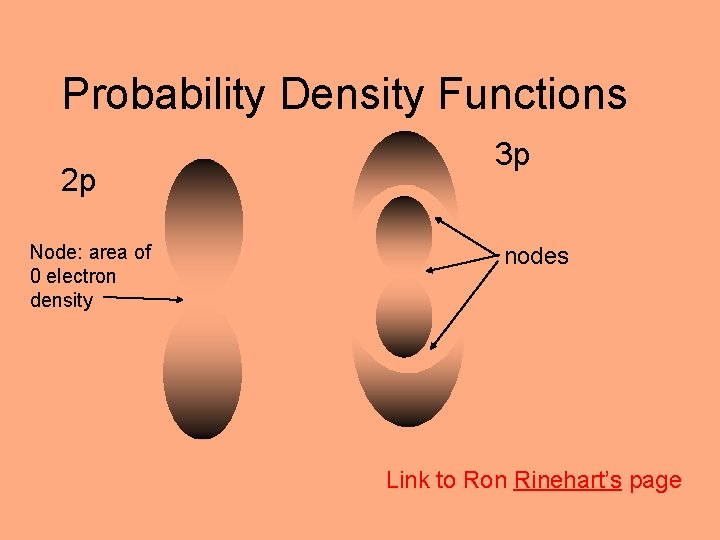
Probability Density Functions (Beyond the Bohr Model) Link to Ron Rinehart’s page energy 2 probability density function s, p, d, f, g 3 s 2 s 1 s Node: area of 0 electron density

Probability Density Functions 2 p Node: area of 0 electron density 3 p nodes Link to Ron Rinehart’s page

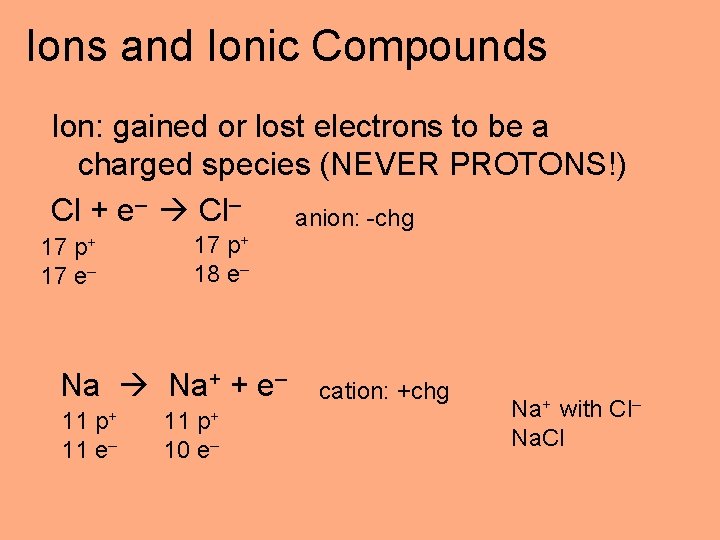
Ions and Ionic Compounds Ion: gained or lost electrons to be a charged species (NEVER PROTONS!) Cl + e– Cl– anion: -chg 17 p+ 17 e– 17 p+ 18 e– Na Na+ + e– p+ 11 11 e– p+ 11 10 e– cation: +chg Na+ with Cl– Na. Cl

Problem 4 Complete the following table of ions: Symbol #p #e 16 18 12 10 Al 3+ Br–

Problem 4 Complete the following table of ions: Symbol #p #e Al 3+ 13 10 Br– 35 36 S 2– 16 18 12 10 Mg 2+