Atoms and Atomic Theory At the end of








- Slides: 17

Atoms and Atomic Theory

At the end of this lesson, you need to draw a diagram for each model showing the evolution of atomic theory: "Plum-Pudding" Model Rutherford Atom Rutherford-Bohr Atom Charge-Cloud Model Quantum Model of the Nucleus Nuclear Shell Model

The Nature of Matter Democritus: Matter is comprised of small individual units (atoms) Aristotle: Matter is comprised of 4 fundamental elements: earth, water, air, and fire Most sided with Aristotle until the Renaissance.

Then in the Early 1800 s John Dalton (1766 – 1844) noticed that substance could be broken down into other substances. Eventually you could not break these down into anything else (atoms). Example. Sugar can be broken into water and carbon dioxide. Water can be broken into oxygen and hydrogen. Carbon dioxide can be broken into carbon and oxygen. Carbon, oxygen and hydrogen cannot be broken down any further. Also, for a particular substance the ratios of atoms produced were always the same. For example: Nitric Acid always gives one “unit” of nitrogen, three “units” of oxygen, and one “unit” of hydrogen.

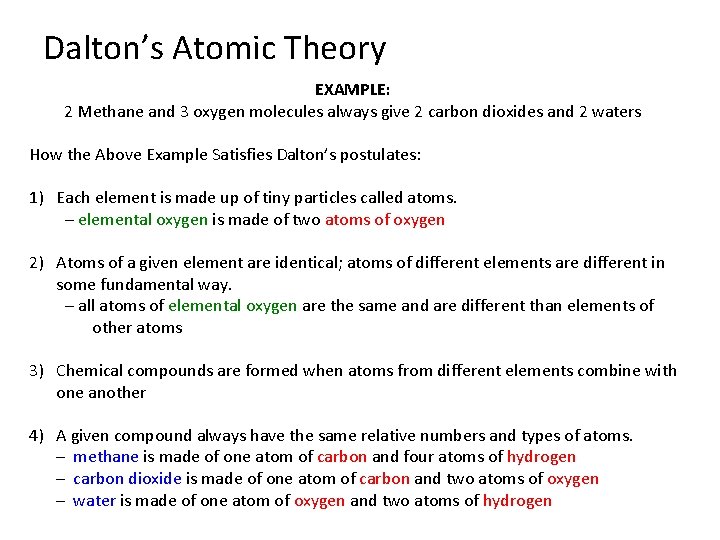
Dalton’s Atomic Theory: Matter is made up of fundamental units called atoms Dalton’s original theory had five postulates: 1) Each element is made up of tiny particles called atoms. (oxygen molecule is made of two atoms of oxygen) 2) Atoms of a given element are identical; atoms of different elements are different in some fundamental way. (MOSTLY TRUE) 3) Chemical compounds are formed when atoms from different elements combine with one another. 4) A given compound always have the same relative numbers and types of atoms. 5) Chemical reactions involve the rearrangement or exchange of atoms within compounds.

Dalton’s Atomic Theory EXAMPLE: 2 Methane and 3 oxygen molecules always give 2 carbon dioxides and 2 waters How the Above Example Satisfies Dalton’s postulates: 1) Each element is made up of tiny particles called atoms. – elemental oxygen is made of two atoms of oxygen 2) Atoms of a given element are identical; atoms of different elements are different in some fundamental way. – all atoms of elemental oxygen are the same and are different than elements of other atoms 3) Chemical compounds are formed when atoms from different elements combine with one another 4) A given compound always have the same relative numbers and types of atoms. – methane is made of one atom of carbon and four atoms of hydrogen – carbon dioxide is made of one atom of carbon and two atoms of oxygen – water is made of one atom of oxygen and two atoms of hydrogen

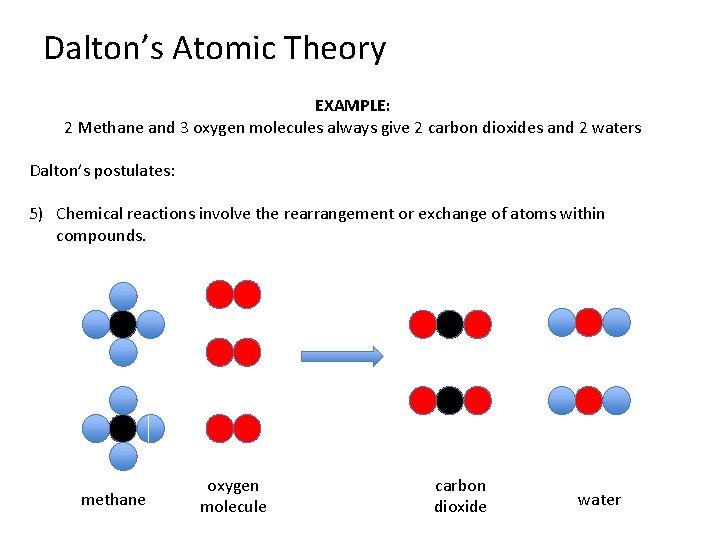
Dalton’s Atomic Theory EXAMPLE: 2 Methane and 3 oxygen molecules always give 2 carbon dioxides and 2 waters Dalton’s postulates: 5) Chemical reactions involve the rearrangement or exchange of atoms within compounds. methane oxygen molecule carbon dioxide water

What Are These Atoms? The existence of atoms remained controversial until ~1900! Even people who believed atoms were real were unsure what they were. Solid Particles? Something more ethereal? Smallest unit of nature? Can they be broken down into smaller parts?

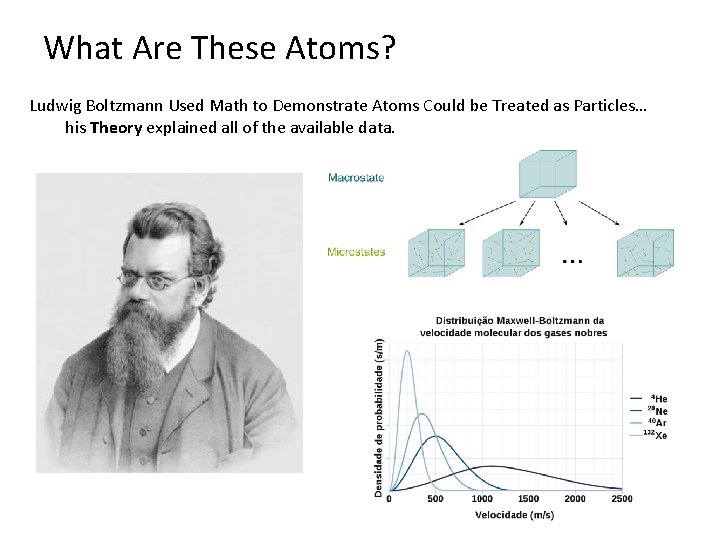
What Are These Atoms? Ludwig Boltzmann Used Math to Demonstrate Atoms Could be Treated as Particles… his Theory explained all of the available data.

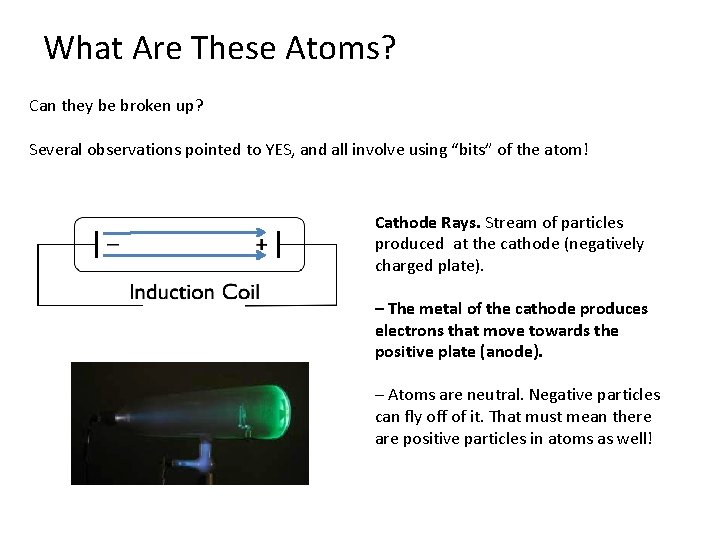
What Are These Atoms? Can they be broken up? Several observations pointed to YES, and all involve using “bits” of the atom! Cathode Rays. Stream of particles produced at the cathode (negatively charged plate). – The metal of the cathode produces electrons that move towards the positive plate (anode). – Atoms are neutral. Negative particles can fly off of it. That must mean there are positive particles in atoms as well!

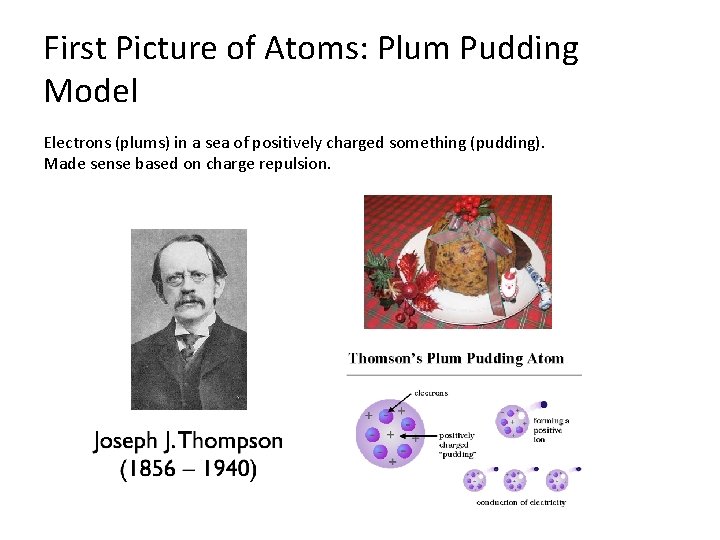
First Picture of Atoms: Plum Pudding Model Electrons (plums) in a sea of positively charged something (pudding). Made sense based on charge repulsion.

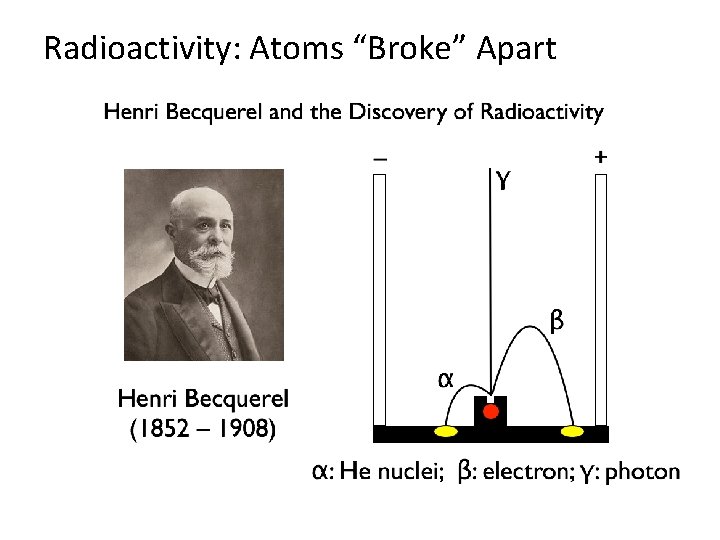
Radioactivity: Atoms “Broke” Apart

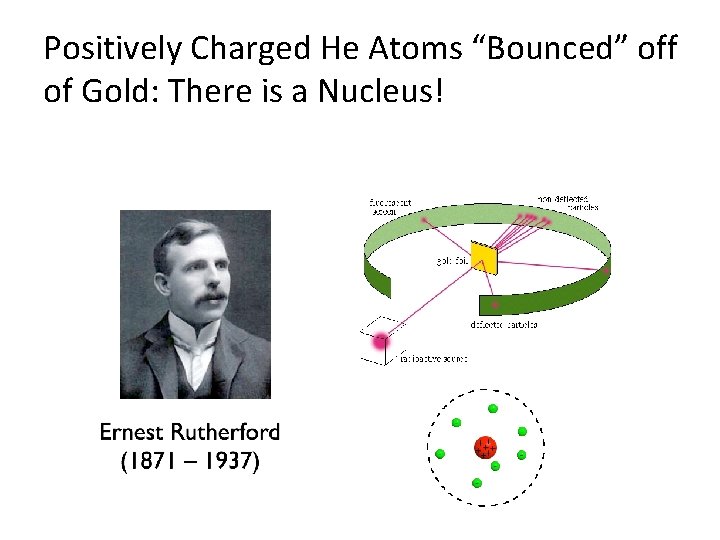
Positively Charged He Atoms “Bounced” off of Gold: There is a Nucleus!


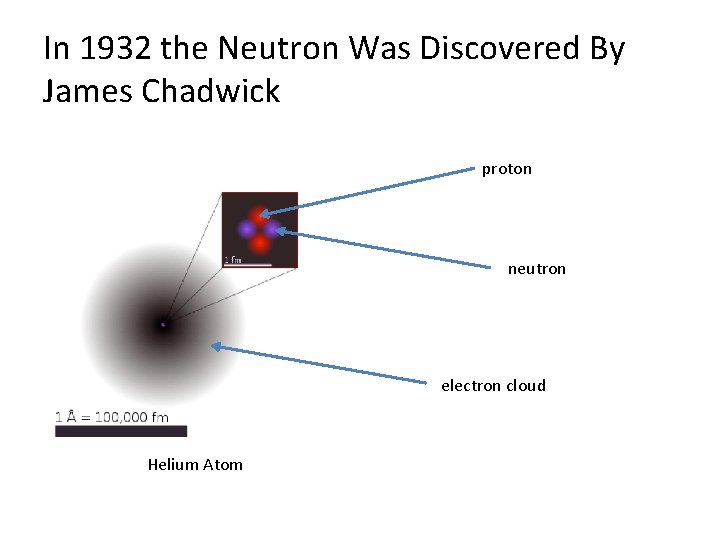
In 1932 the Neutron Was Discovered By James Chadwick proton neutron electron cloud Helium Atom

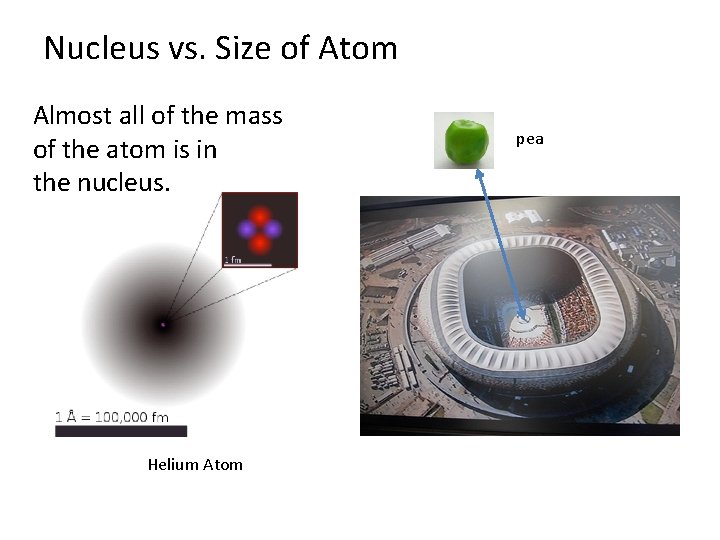
Nucleus vs. Size of Atom Almost all of the mass of the atom is in the nucleus. Helium Atom pea

Draw a diagram for each model showing the evolution of atomic theory: • • • "Plum-Pudding" Model Based on the work of J. J. Thomson, who discovered that electrons have a negative charge (about 1897), this model presents the atom as a collection of negatively charged electrons mixed with positively charged particles. Rutherford Atom Based on the work of Ernest Rutherford in 1911, this model is the first to establish the nucleus in the center of the atom, with negatively charged electrons orbiting it, as planets orbit the sun. Rutherford-Bohr Atom In 1913, Niels Bohr improved upon the Rutherford model by stating that electrons travel the nucleus in a fixed orbit. At times, electrons can move from one energy level to another. Charge-Cloud Model With the emergence of Werner Heisenberg's Uncertainty Principle, which states that it is impossible to know both the location and speed of an electron at the same time, scientists developed what is known as the charge-cloud model. According to this model, electrons move around the nucleus, but no attempt is made to show the orbital paths of the electrons. The electrons are depicted within a cloud to indicate that they are traveling at high speeds. Quantum Model of the Nucleus In 1930, James Chadwick discovered the neutron, which was found to have the same mass as the proton. With this discovery, it became clear that the mass of an atom came from the nucleus. Nuclear Shell Model This model is used to describe the behavior of electrons orbiting the nucleus of an atom. Electrons arrange themselves in shells around the atom, with the outermost shell called the valence shell. If this shell has eight electrons, it is considered stable. During chemical reactions, the valence shell will gain or lose electrons to increase its stability.
 Periodic table regents
Periodic table regents The atoms family atomic math challenge answer key
The atoms family atomic math challenge answer key Reference table of atomic radii
Reference table of atomic radii Atom family song
Atom family song Atoms family atomic math challenge
Atoms family atomic math challenge The atoms family worksheet
The atoms family worksheet The atoms family atomic math challenge
The atoms family atomic math challenge Is atomic mass and relative atomic mass the same
Is atomic mass and relative atomic mass the same How do you calculate atomic mass
How do you calculate atomic mass Distinguish between mass number and atomic mass.
Distinguish between mass number and atomic mass. Trends of the periodic table
Trends of the periodic table Atomic radius in a periodic table
Atomic radius in a periodic table Atomic number vs atomic radius
Atomic number vs atomic radius Electrons in atoms section 2 quantum theory and the atom
Electrons in atoms section 2 quantum theory and the atom Electrons in atoms section 2 quantum theory and the atom
Electrons in atoms section 2 quantum theory and the atom Quantum theory and the electronic structure of atoms
Quantum theory and the electronic structure of atoms Front end and back end in compiler design
Front end and back end in compiler design Front-end and back-end of compiler
Front-end and back-end of compiler