ATOMS AND ATOMIC STRUCTURE Atom Nucleus Proton Neutron





- Slides: 19

ATOMS AND ATOMIC STRUCTURE Atom Nucleus Proton Neutron Electron

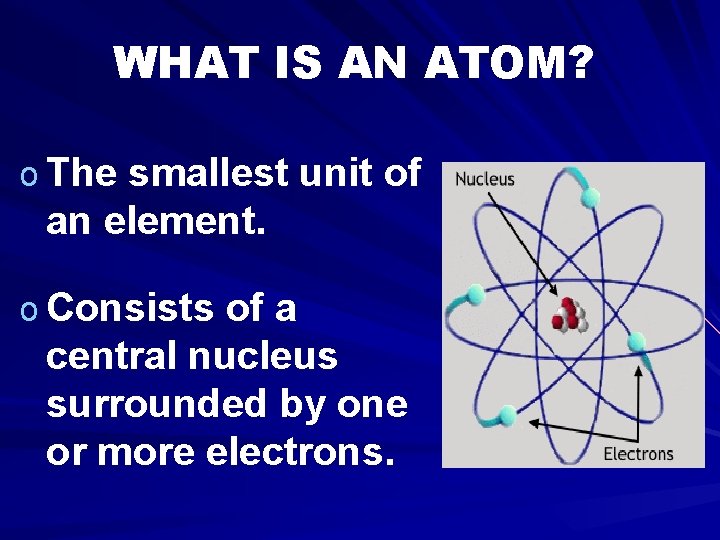
WHAT IS AN ATOM? o The smallest unit of an element. o Consists of a central nucleus surrounded by one or more electrons.

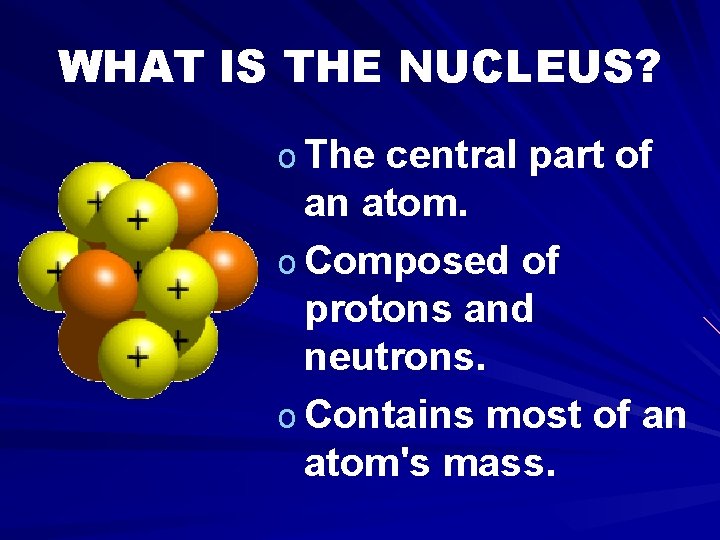
WHAT IS THE NUCLEUS? o The central part of an atom. o Composed of protons and neutrons. o Contains most of an atom's mass.

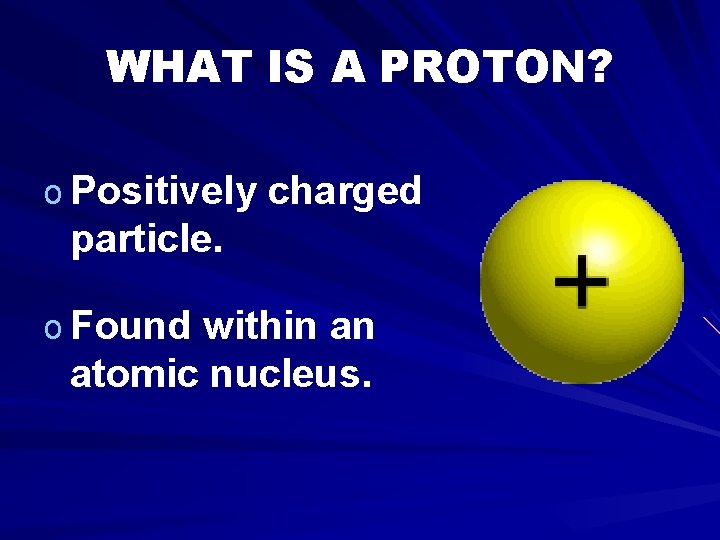
WHAT IS A PROTON? o Positively charged particle. o Found within an atomic nucleus.

WHAT IS A NEUTRON? o Uncharged particle. o Found within an atomic nucleus.

WHAT IS AN ELECTRON? o Negatively charged particle. o Located in shells that surround an atom's nucleus.

THE BOHR MODEL OF THE ATOM 1913 Niels Bohr studied under Rutherford at the Victoria University in Manchester. Bohr refined Rutherford's idea by adding that the electrons were in orbits. Rather like planets orbiting the sun. With each orbit only able to contain a set number of electrons.

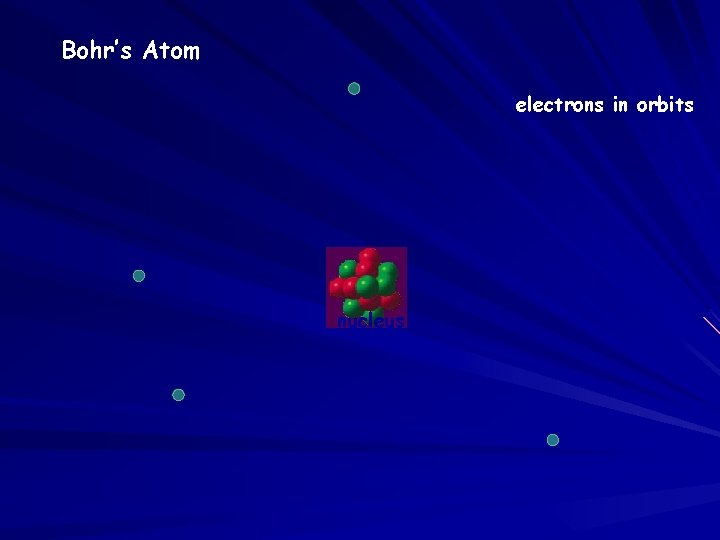
Bohr’s Atom electrons in orbits nucleus

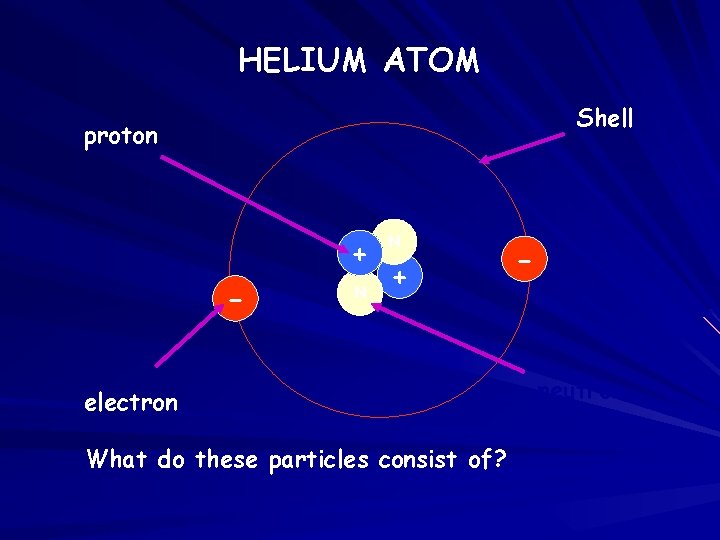
HELIUM ATOM Shell proton + - N N + electron What do these particles consist of? - neutron

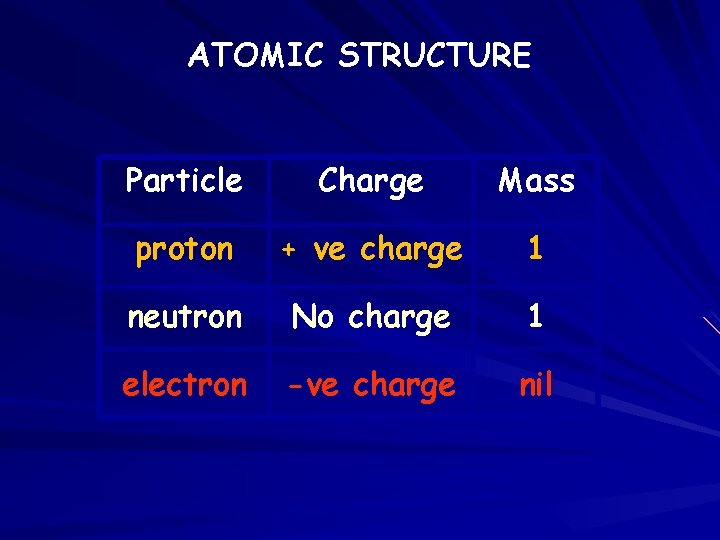
ATOMIC STRUCTURE Particle Charge Mass proton + ve charge 1 neutron No charge 1 electron -ve charge nil

ATOMIC STRUCTURE 4 2 He Atomic number of Atomic mass the number of protons in an atom the number of protons and electrons = number of protons neutrons in an atom

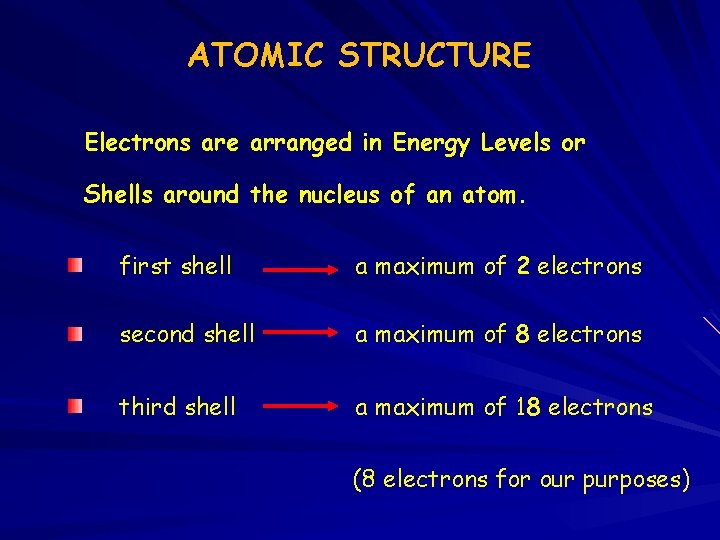
ATOMIC STRUCTURE Electrons are arranged in Energy Levels or Shells around the nucleus of an atom. first shell a maximum of 2 electrons second shell a maximum of 8 electrons third shell a maximum of 18 electrons (8 electrons for our purposes)

ATOMIC STRUCTURE There are two ways to represent the atomic structure of an element or compound; 1. Electronic Configuration 2. Dot & Cross Diagrams

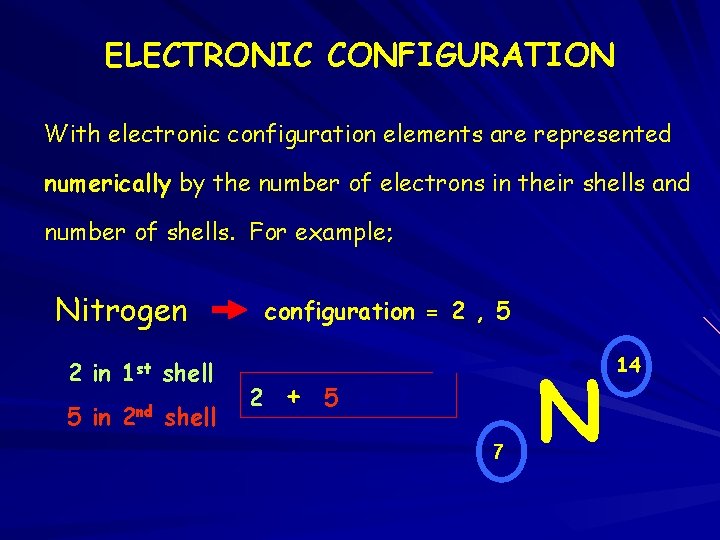
ELECTRONIC CONFIGURATION With electronic configuration elements are represented numerically by the number of electrons in their shells and number of shells. For example; Nitrogen 2 in 1 st shell 5 in 2 nd shell configuration = 2 , 5 2 + 5 = 7 7 N 14 14

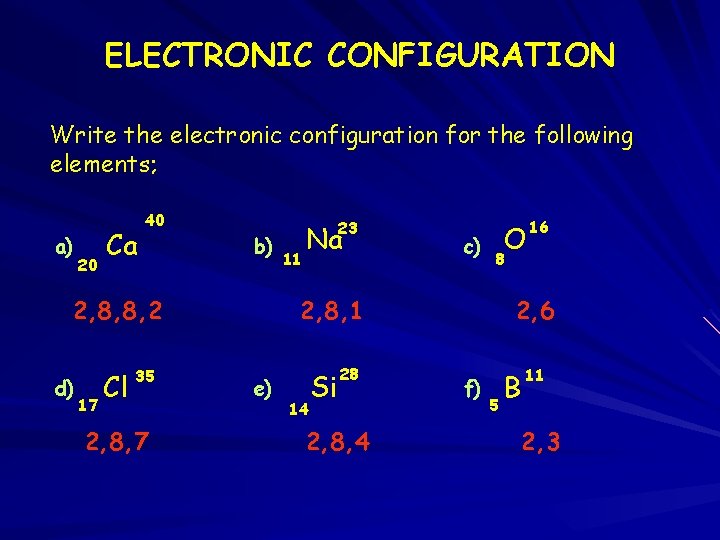
ELECTRONIC CONFIGURATION Write the electronic configuration for the following elements; a) 20 Ca 40 b) 2, 8, 8, 2 d) 17 Cl 35 2, 8, 7 23 11 Na c) O 8 2, 8, 1 e) 14 Si 28 2, 8, 4 16 2, 6 f) 5 B 11 2, 3

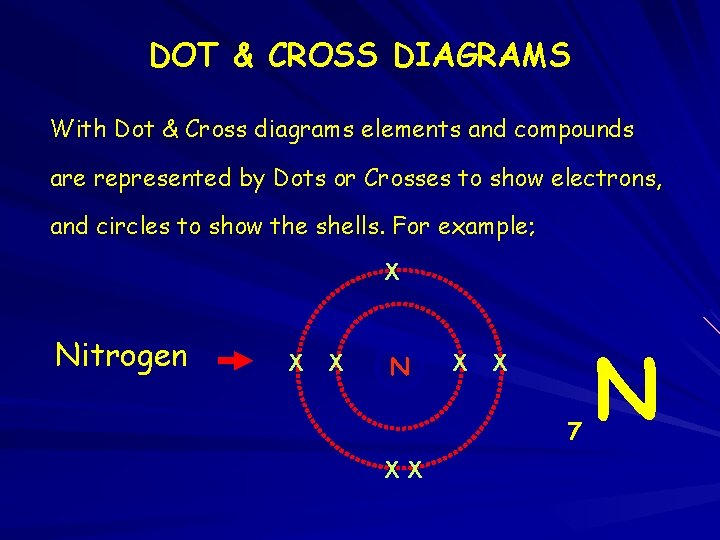
DOT & CROSS DIAGRAMS With Dot & Cross diagrams elements and compounds are represented by Dots or Crosses to show electrons, and circles to show the shells. For example; X Nitrogen X X N X X 7 XX N 14

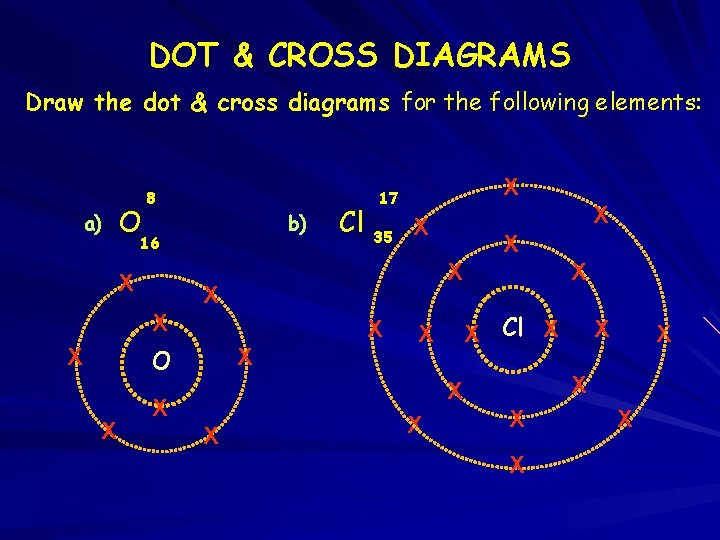
DOT & CROSS DIAGRAMS Draw the dot & cross diagrams for the following elements: a) O 8 b) 16 X X X O X 35 X X X Cl X 17 X X X Cl X X X

SUMMARY 1. The Atomic Number of an atom = number of protons in the nucleus. 2. The Atomic Mass of an atom = number of Protons + Neutrons in the nucleus. 3. The number of Protons = Number of Electrons. 4. Electrons orbit the nucleus in shells. 5. Each shell can only carry a set number of electrons.
