ATOMS All matter is made up of atoms











- Slides: 20

ATOMS

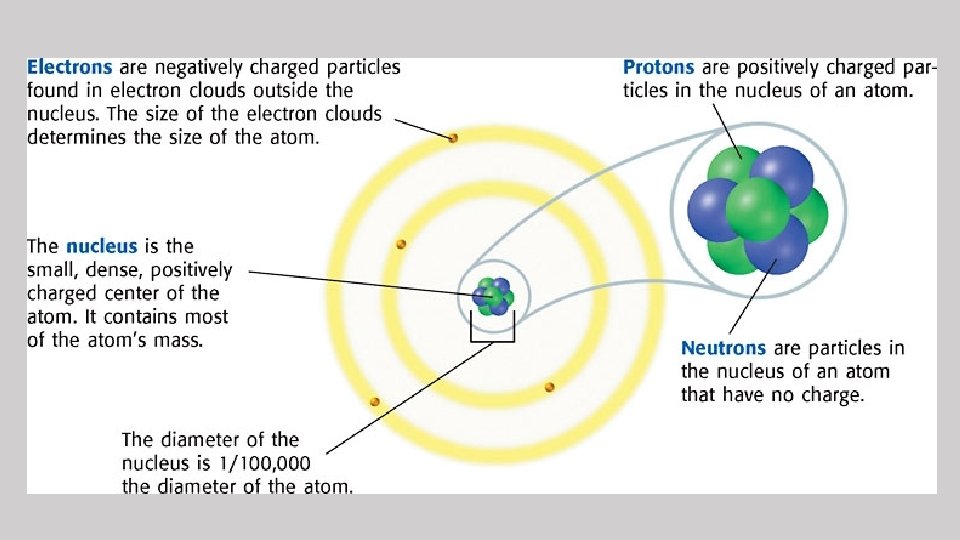
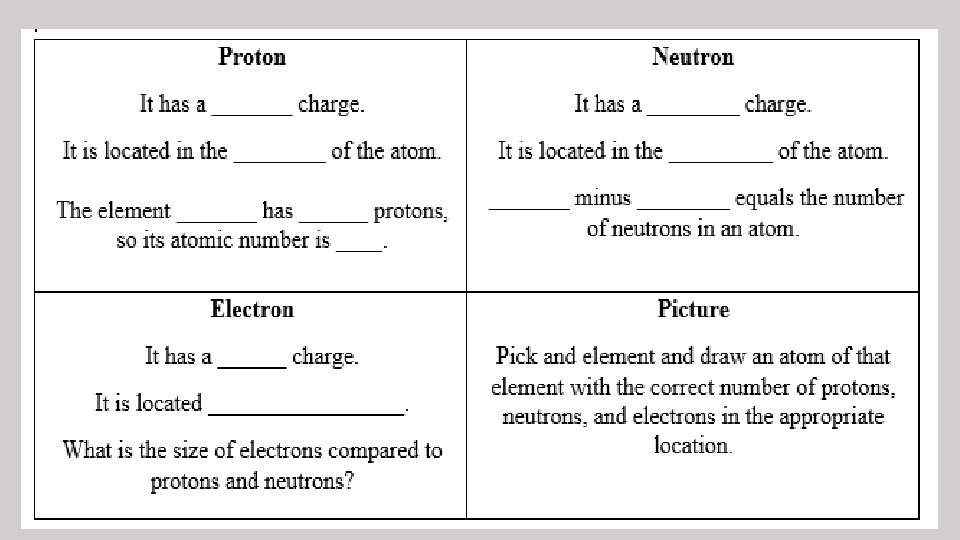
• All matter is made up of atoms. • An atom is the smallest unit of an element that has all the properties of the element. • Atoms are made up of protons, neutrons, and electrons.

Protons • Positive charge • Located in the nucleus • Elements are organized on the Periodic Table based on their atomic number (number of protons) Ex: Neon has 10 protons so its atomic number is 10, and it is the 10 th element on the Periodic Table.

Neutrons • Neutral charge (no charge) • Located in the nucleus • Similar in size to protons

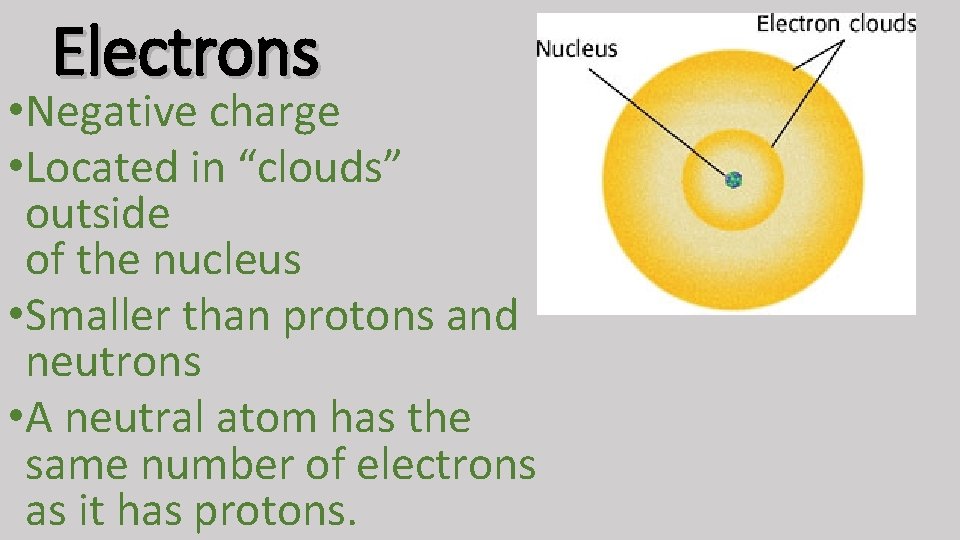
Electrons • Negative charge • Located in “clouds” outside of the nucleus • Smaller than protons and neutrons • A neutral atom has the same number of electrons as it has protons.



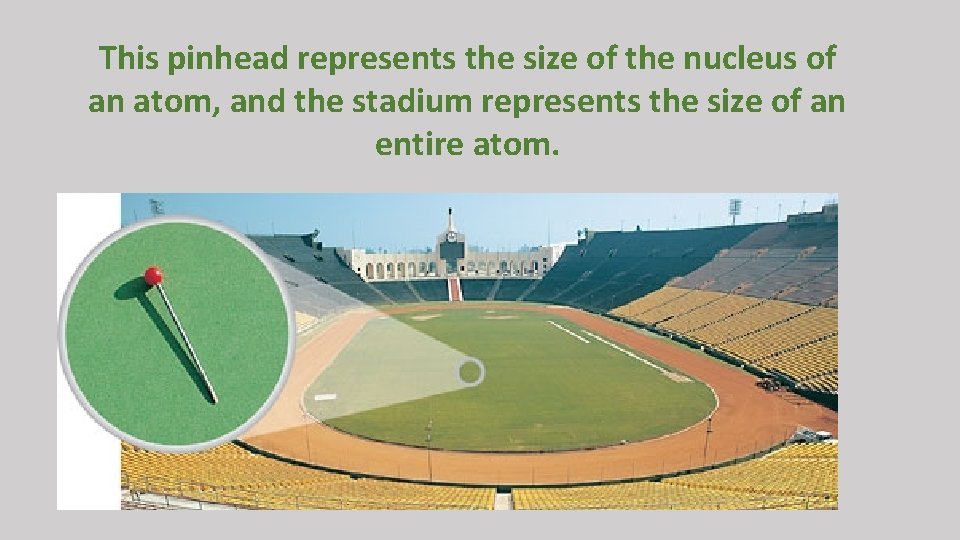
This pinhead represents the size of the nucleus of an atom, and the stadium represents the size of an entire atom.

Atomic Theory • Began in ancient Greece and continues today. • As technology has improved, we know more about atoms.

• Democritus (Greek philosopher (440 B. C. ) believed: 1. If you keep cutting something in half, eventually you will have a particle that can’t be cut. 2. Atom (atomos)- “unable to be divided” 3. thought atoms were small and hard

• Dalton (1700 s chemist/teacher) believed: 1. Everything is made of atoms. 2. Atoms can’t be created, destroyed, or divided. 3. Atoms of the same element are exactly alike and atoms of different elements are different. 4. Atoms combine to make new substances.

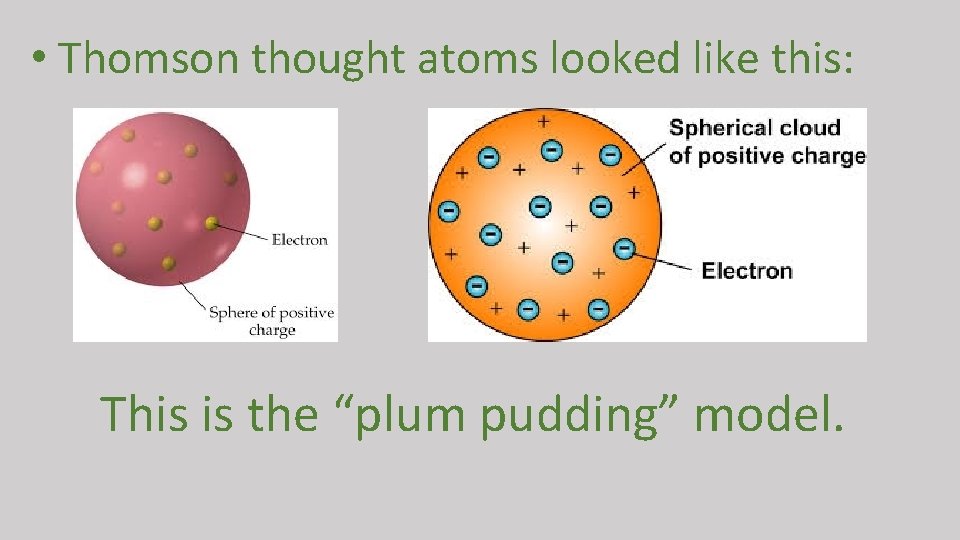
• J. J. Thomson (1897 British scientist): 1. discovered electrons 2. thought atoms were a positive sphere with negative dots

• Thomson thought atoms looked like this: This is the “plum pudding” model.

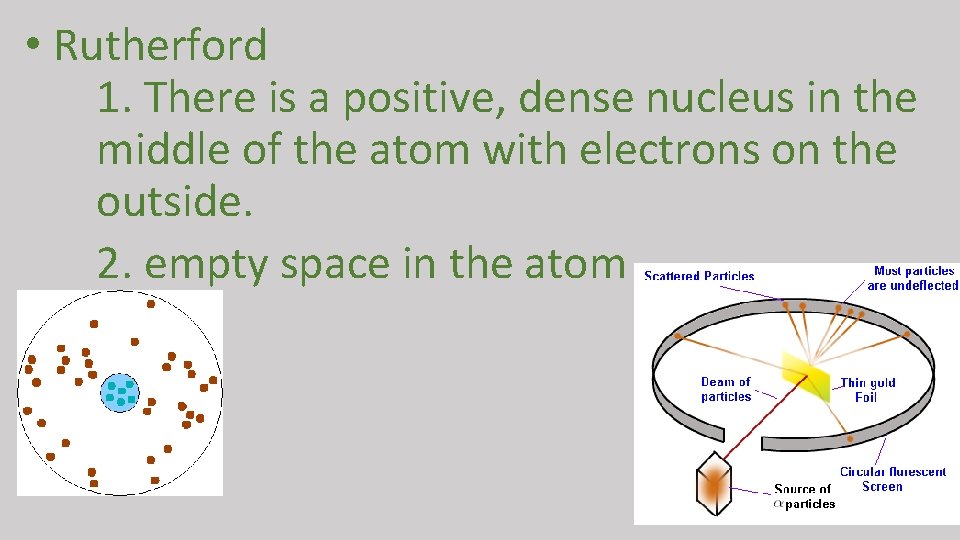
• Rutherford 1. There is a positive, dense nucleus in the middle of the atom with electrons on the outside. 2. empty space in the atom

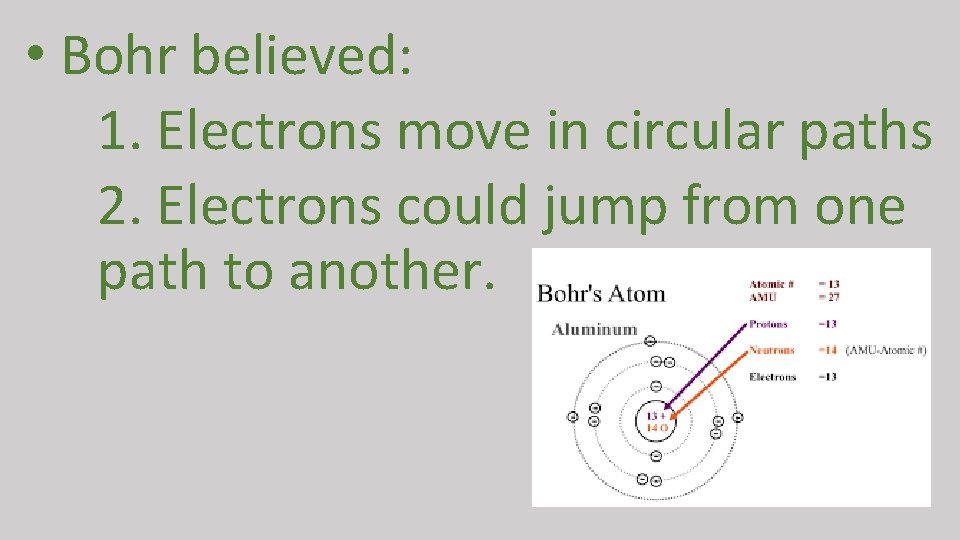
• Bohr believed: 1. Electrons move in circular paths 2. Electrons could jump from one path to another.

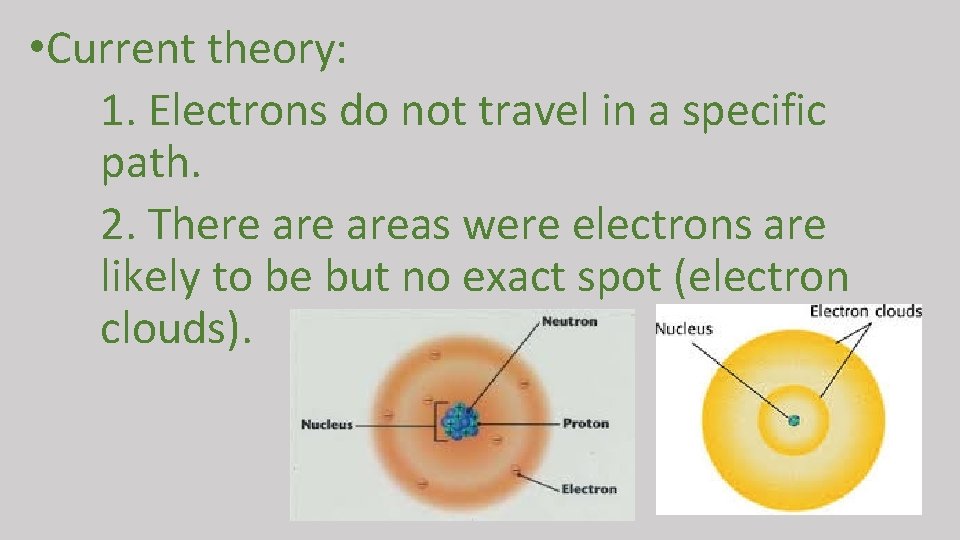
• Current theory: 1. Electrons do not travel in a specific path. 2. There areas were electrons are likely to be but no exact spot (electron clouds).

Isotopes • same number of protons but different number of neutrons • We tell isotopes apart based on their mass number (the number of protons plus the number of neutrons). • Mass number – atomic number = number of neutrons • Isotopes are named based on mass number. Ex: Boron-10 has 5 protons and 5 neutrons Boron-11 has 5 protons and 6 neutrons
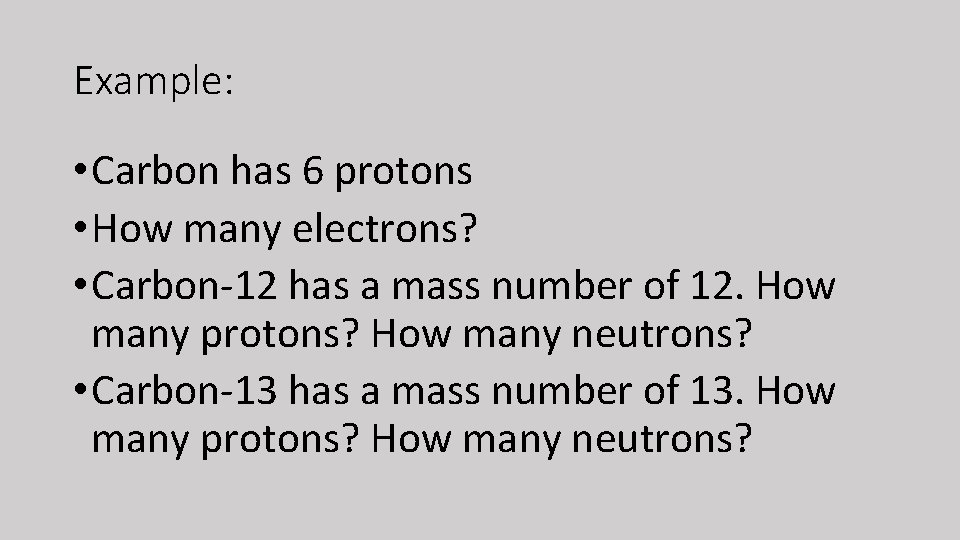
Example: • Carbon has 6 protons • How many electrons? • Carbon-12 has a mass number of 12. How many protons? How many neutrons? • Carbon-13 has a mass number of 13. How many protons? How many neutrons?

Atomic number? Mass number? Protons? Electrons? Neutrons?
