Atomic Timeline Development of the Atomic Theory Democtritus




- Slides: 11

“Atomic Timeline” Development of the Atomic Theory

Democtritus • Greek • First coined the term “Atom” which means invisible and indivisible. • Came up with theory while trying to starve himself to death. • Aristotle had opposing theory which was believed for 2000 years

Boyle & Newton Robert Boyle • Began ‘experimental chemistry’ • Studied gases • Worked with Newton to bring alchemy out of the “dark ages”. Eventually became Chemistry. Isaac Newton • Isaac Newton was one of the first people to propose a “mechanical universe. ” He believed that the universe is made up of small solid masses that are constantly in motion. This idea is known as “The Mechanical Universe” idea. • Wrote article “Skeptycal Chemyst”

John Dalton • John Dalton formulated the Atomic Theory which states that: atoms of an element are different than atoms of other elements; atoms of one element are the same; that atoms of different elements can be combined; that atoms cannot be divided or separated; and that elements are made of tiny particles called atoms. • Used actual experimental work from Antoine Lavoisier (Law of conservation of Mass) and Joseph Proust (Law of Definite Proportions) to support his theory.

JJ Thomson • Experimented with Cathode rays. • Based on the work that he did, he discovered electrons, the first subatomic particle • He also developed a model of the atom called the “plum pudding” model. • Because of Thomson’s work, Dalton’s theory was adjusted.

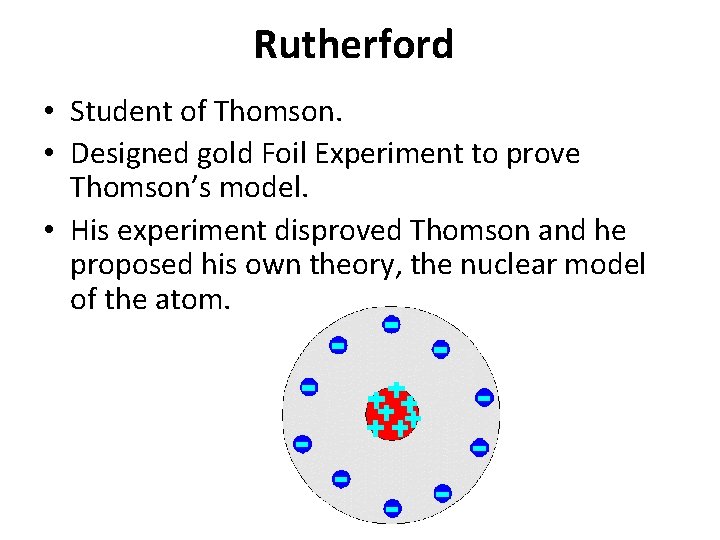
Rutherford • Student of Thomson. • Designed gold Foil Experiment to prove Thomson’s model. • His experiment disproved Thomson and he proposed his own theory, the nuclear model of the atom.

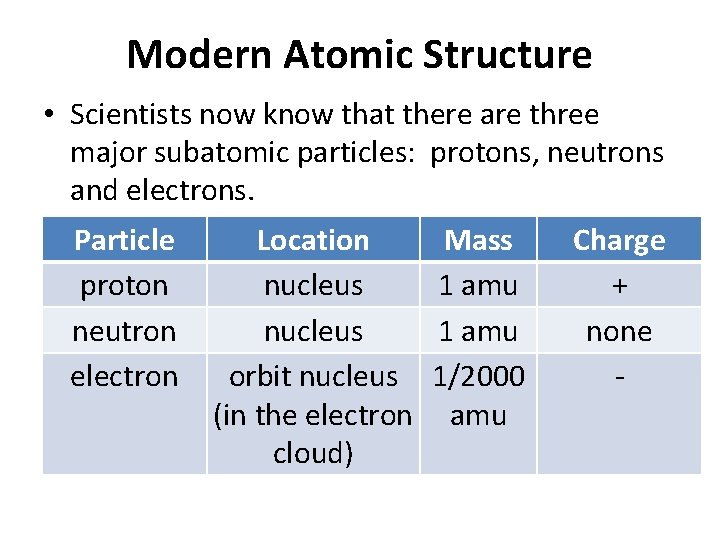
Modern Atomic Structure • Scientists now know that there are three major subatomic particles: protons, neutrons and electrons. Particle proton neutron electron Location nucleus orbit nucleus (in the electron cloud) Mass 1 amu 1/2000 amu Charge + none -

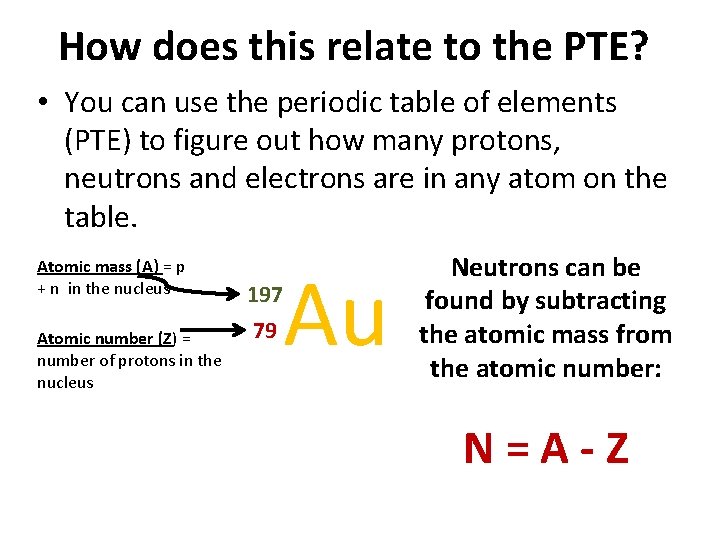
How does this relate to the PTE? • You can use the periodic table of elements (PTE) to figure out how many protons, neutrons and electrons are in any atom on the table. Atomic mass (A) = p + n in the nucleus Atomic number (Z) = number of protons in the nucleus Au 197 79 Neutrons can be found by subtracting the atomic mass from the atomic number: N=A-Z

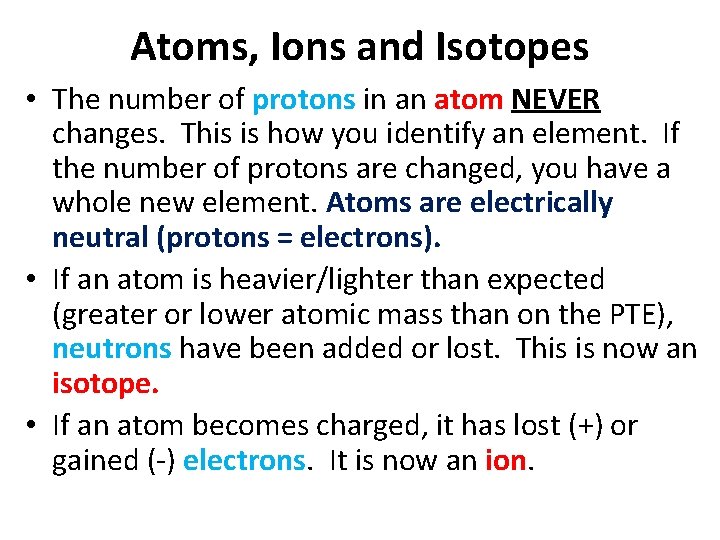
Atoms, Ions and Isotopes • The number of protons in an atom NEVER changes. This is how you identify an element. If the number of protons are changed, you have a whole new element. Atoms are electrically neutral (protons = electrons). • If an atom is heavier/lighter than expected (greater or lower atomic mass than on the PTE), neutrons have been added or lost. This is now an isotope. • If an atom becomes charged, it has lost (+) or gained (-) electrons. It is now an ion.

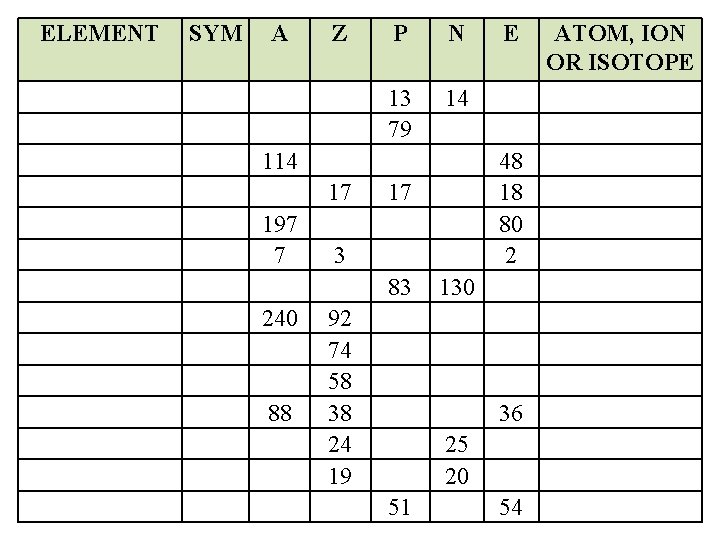
ELEMENT SYM A Z P N 13 79 14 17 197 7 240 88 48 18 80 2 17 3 83 92 74 58 38 24 19 E 130 36 25 20 51 54 ATOM, ION OR ISOTOPE

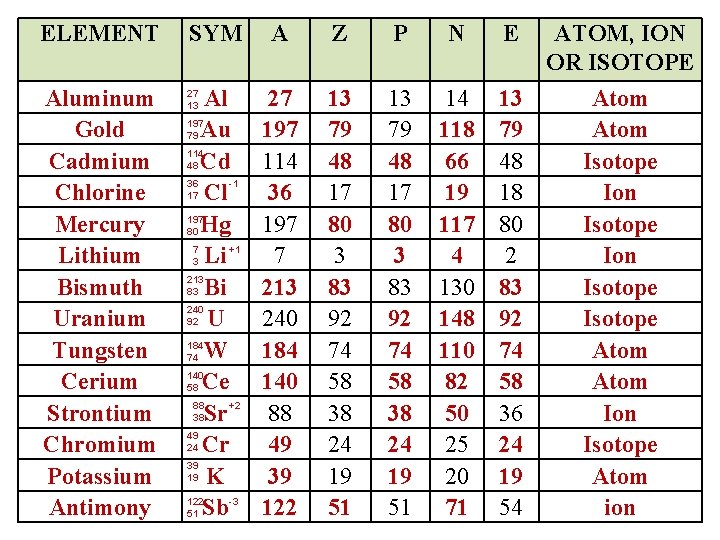
ELEMENT Aluminum Gold Cadmium Chlorine Mercury Lithium Bismuth Uranium Tungsten Cerium Strontium Chromium Potassium Antimony SYM A Z P N E Al 197 79 Au 114 48 Cd 36 -1 17 Cl 197 80 Hg 7 +1 3 Li 213 83 Bi 240 92 U 184 74 W 140 58 Ce 88 +2 38 Sr 49 24 Cr 39 19 K 122 -3 51 Sb 27 197 114 36 197 7 213 240 184 140 88 49 39 122 13 79 48 17 80 3 83 92 74 58 38 24 19 51 14 118 66 19 117 4 130 148 110 82 50 25 20 71 13 79 48 18 80 2 83 92 74 58 36 24 19 54 27 13 ATOM, ION OR ISOTOPE Atom Isotope Ion Isotope Atom ion