Atomic Theory Radiation Chapter 7 286 325 Superheroes











- Slides: 23

Atomic Theory Radiation Chapter 7 286 - 325

Superheroes – The Hulk

Superheroes – Spider Man

The Atomic Patrol

7. 1 - Radiation: high energy rays and particles emitted by radioactive sources. Various levels of radiation can be found all around us, emitted from the ground or atmosphere (space – sun and stars). Radioactivity: release of high-energy particles and rays of energy from a substance as a result of changes in the nuclei of its atoms. Radioactivity can be useful! Eg: Medicine – treatment and diagnosis ( x-rays, radiotherapy etc), generating electricity etc.

Radiation Natural Background Radiation: high energy, fast moving particles or waves found in our environment. Types of Radiation: 1. 2. 3. 4. 5. Radio waves Microwaves – radar, cell phones Infrared Rays – sun, fire, living creatures; felt as heat Visible Light – light we can see with our eyes Ultraviolet Rays – helps us produce Vitamin D but in excess can cause a sunburn! Radiation is invisible to the naked eye except for: Visible Light

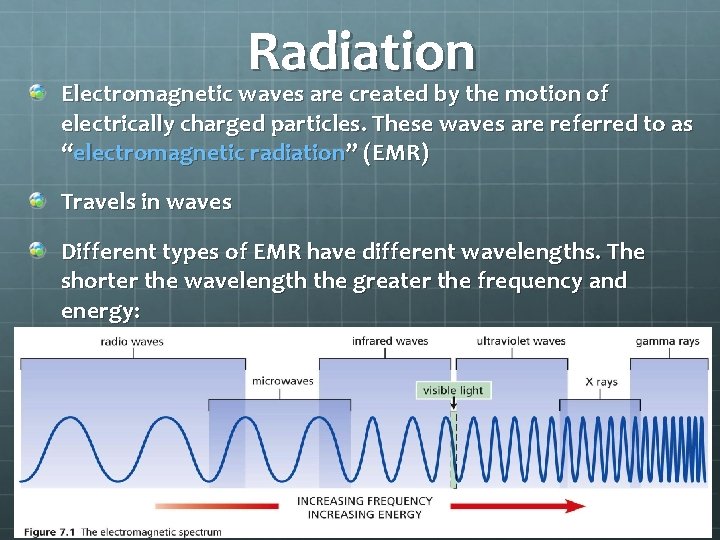
Radiation Electromagnetic waves are created by the motion of electrically charged particles. These waves are referred to as “electromagnetic radiation” (EMR) Travels in waves Different types of EMR have different wavelengths. The shorter the wavelength the greater the frequency and energy:

Marie Curie


Isotopes Isotope: different atoms of an element that have the same number of protons but different number of neutrons. Therefore, the mass number will vary from one isotope to another but an atoms number of electrons and protons do not change.

Isotopes Recall: an atom’s mass is made up of the number of neutrons and protons within an atoms nucleus. Calculation for the # of neutrons: Neutrons = mass number – atomic number

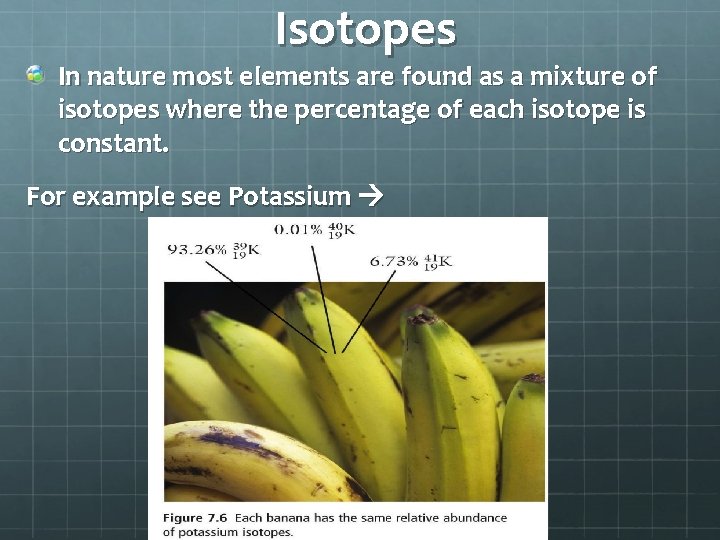
Isotopes In nature most elements are found as a mixture of isotopes where the percentage of each isotope is constant. For example see Potassium

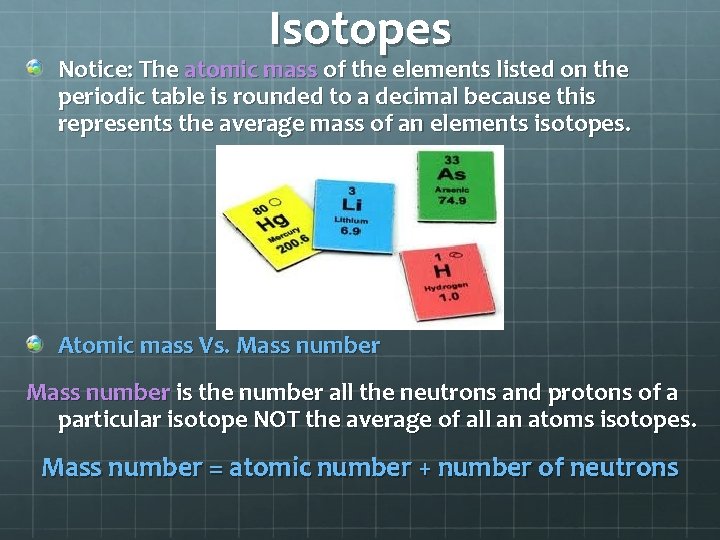
Isotopes Notice: The atomic mass of the elements listed on the periodic table is rounded to a decimal because this represents the average mass of an elements isotopes. Atomic mass Vs. Mass number is the number all the neutrons and protons of a particular isotope NOT the average of all an atoms isotopes. Mass number = atomic number + number of neutrons

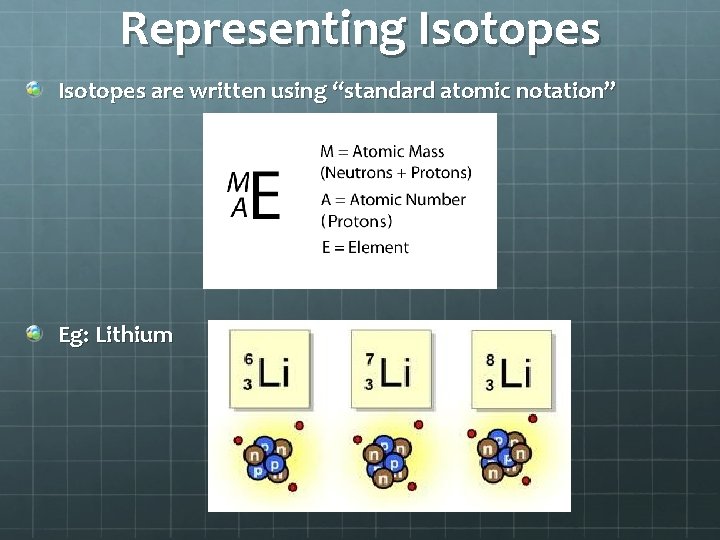
Representing Isotopes are written using “standard atomic notation” Eg: Lithium

Radioactive Decay Some isotopes have unstable nuclei. Process where an unstable isotope gains stability is called Radioactive Decay: When unstable nuclei lose energy by emitting radiation Radioactive decay can turn an atom of one element into an atom of a different element Radioisotopes: are isotopes that are capable of radioactive decay.

Isotopes and Decay

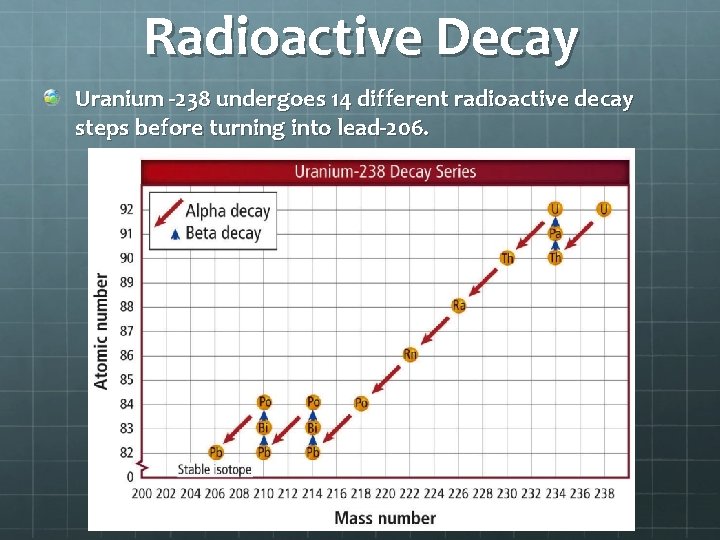
Radioactive Decay Uranium -238 undergoes 14 different radioactive decay steps before turning into lead-206.

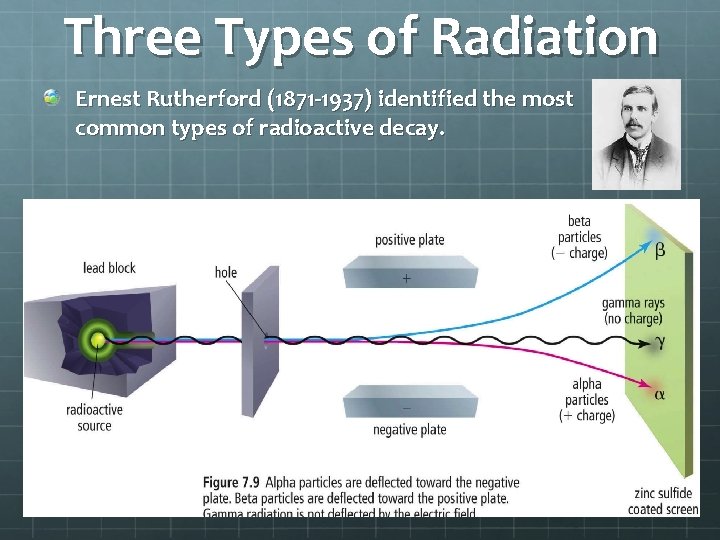
Three Types of Radiation Ernest Rutherford (1871 -1937) identified the most common types of radioactive decay.

Rutherford's Experiment

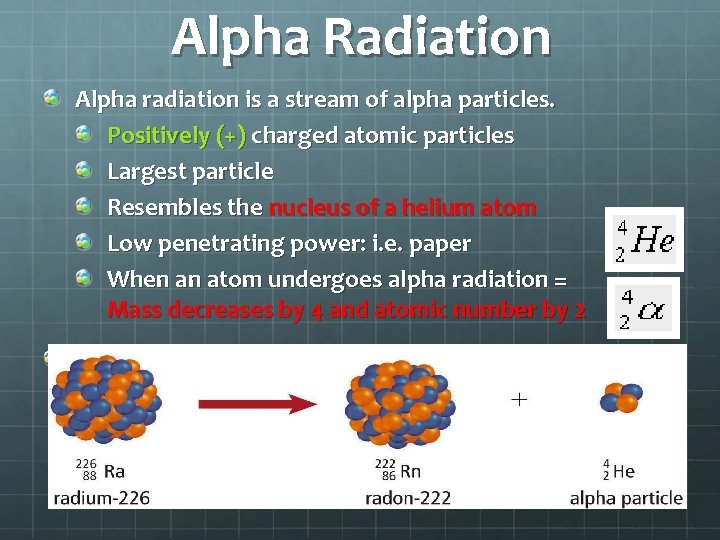
Alpha Radiation Alpha radiation is a stream of alpha particles. Positively (+) charged atomic particles Largest particle Resembles the nucleus of a helium atom Low penetrating power: i. e. paper When an atom undergoes alpha radiation = Mass decreases by 4 and atomic number by 2 Alpha particles are represented by these symbols:

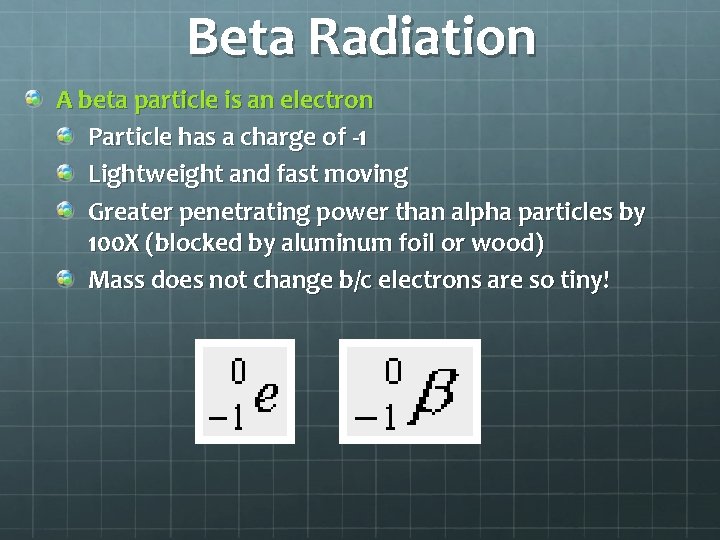
Beta Radiation A beta particle is an electron Particle has a charge of -1 Lightweight and fast moving Greater penetrating power than alpha particles by 100 X (blocked by aluminum foil or wood) Mass does not change b/c electrons are so tiny!

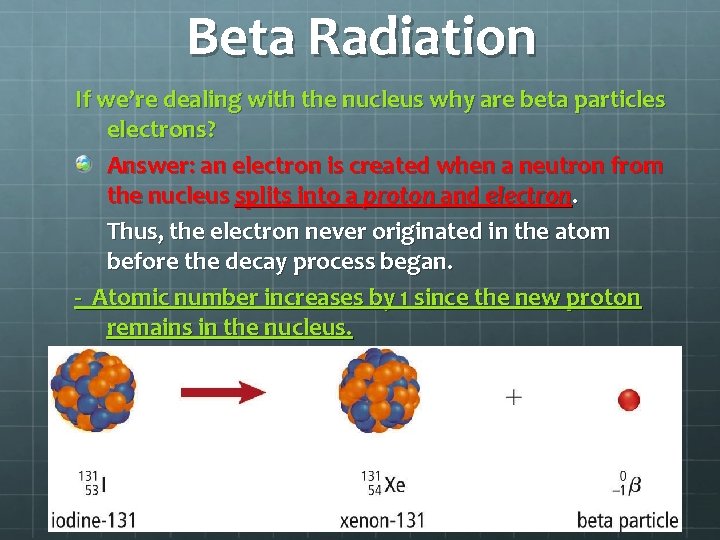
Beta Radiation If we’re dealing with the nucleus why are beta particles electrons? Answer: an electron is created when a neutron from the nucleus splits into a proton and electron. Thus, the electron never originated in the atom before the decay process began. - Atomic number increases by 1 since the new proton remains in the nucleus.

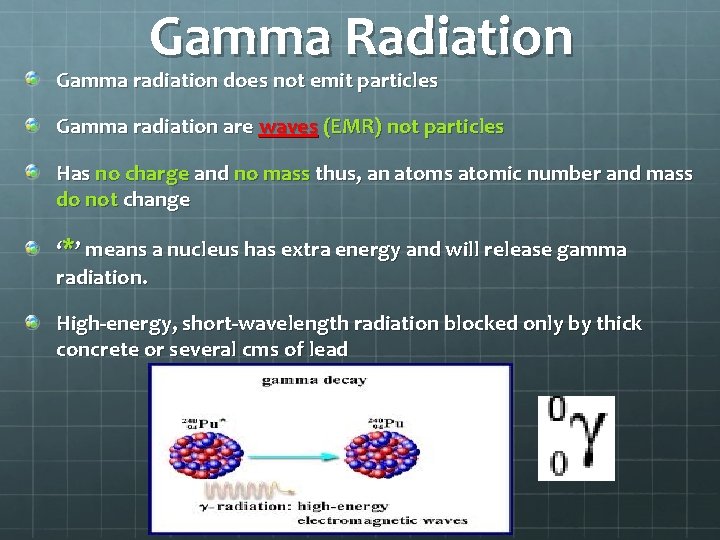
Gamma Radiation Gamma radiation does not emit particles Gamma radiation are waves (EMR) not particles Has no charge and no mass thus, an atoms atomic number and mass do not change ‘*’ means a nucleus has extra energy and will release gamma radiation. High-energy, short-wavelength radiation blocked only by thick concrete or several cms of lead