Atomic Theory Quantum Model of the Atom Review
















- Slides: 49

Atomic Theory Quantum Model of the Atom

Review • J. J. Thompson determined all matter contains electrons with cathode ray tube experiments. • Rutherford demonstrated that atoms contain mostly empty space by bombarding metals with alpha particles (helium nuclei) • Previously we talked about the blueberry muffin model of an atom and Rutherford’s planetary model.

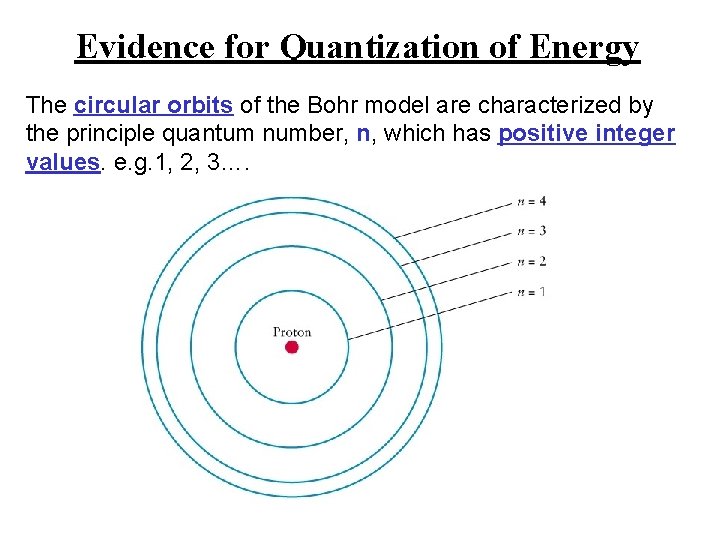
Evidence for Quantization of Energy The circular orbits of the Bohr model are characterized by the principle quantum number, n, which has positive integer values. e. g. 1, 2, 3….

About Energy is anything that has the capacity to do work. Energy Examples: Food; how much energy for a heart beat? Gasoline; how many $ goes to heat and how much to work? Dynamite; what can it move? Electricity; what can it move? Apple on a tree; what can it move?

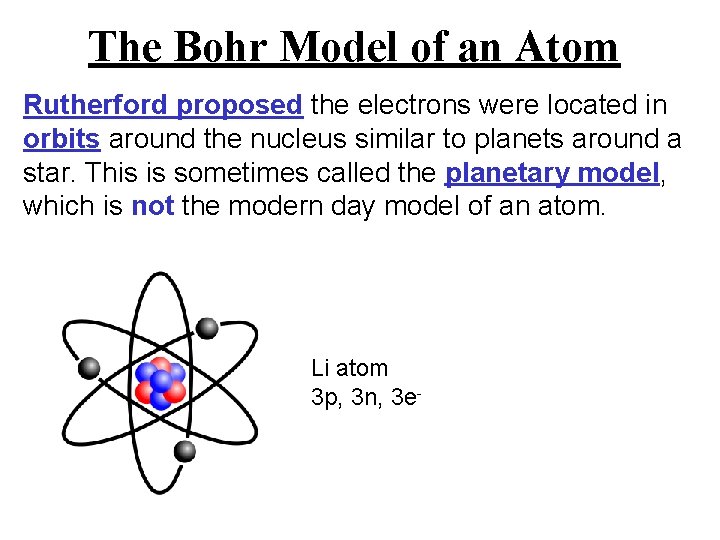
The Bohr Model of an Atom Rutherford proposed the electrons were located in orbits around the nucleus similar to planets around a star. This is sometimes called the planetary model, which is not the modern day model of an atom. Li atom 3 p, 3 n, 3 e-

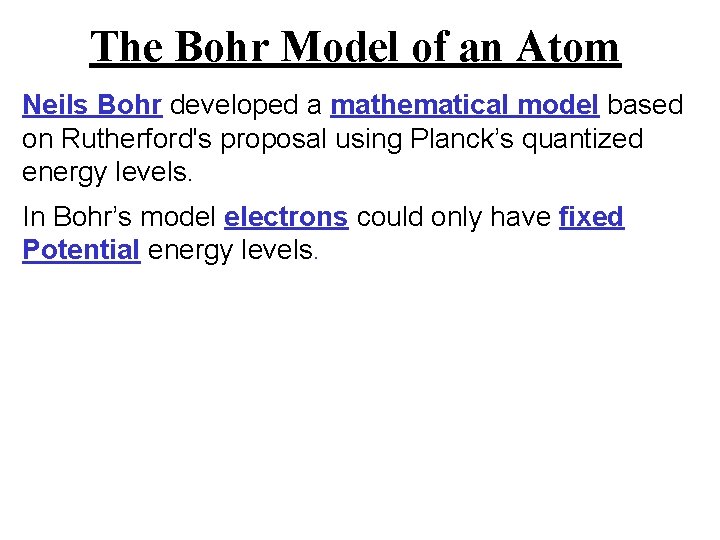
The Bohr Model of an Atom Neils Bohr developed a mathematical model based on Rutherford's proposal using Planck’s quantized energy levels. In Bohr’s model electrons could only have fixed Potential energy levels.

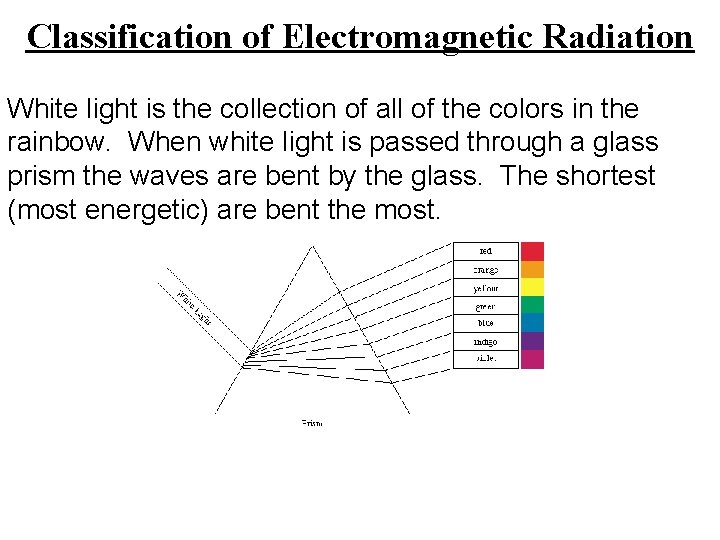
Classification of Electromagnetic Radiation White light is the collection of all of the colors in the rainbow. When white light is passed through a glass prism the waves are bent by the glass. The shortest (most energetic) are bent the most.

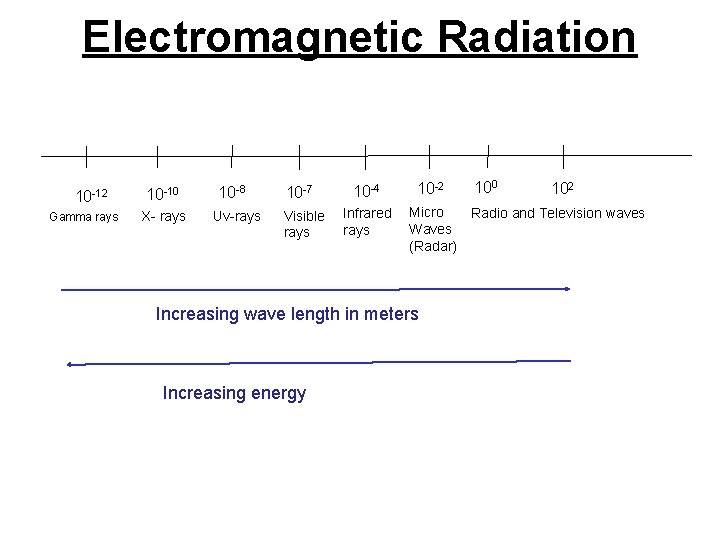
Electromagnetic Radiation 10 -12 Gamma rays 10 -10 X- rays 10 -8 Uv-rays 10 -7 Visible rays 10 -4 10 -2 Infrared rays Micro Waves (Radar) Increasing wave length in meters Increasing energy 100 102 Radio and Television waves

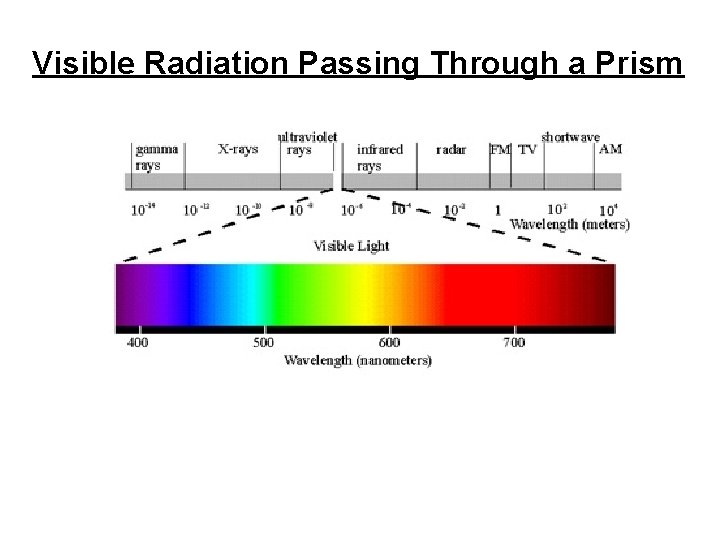
Visible Radiation Passing Through a Prism

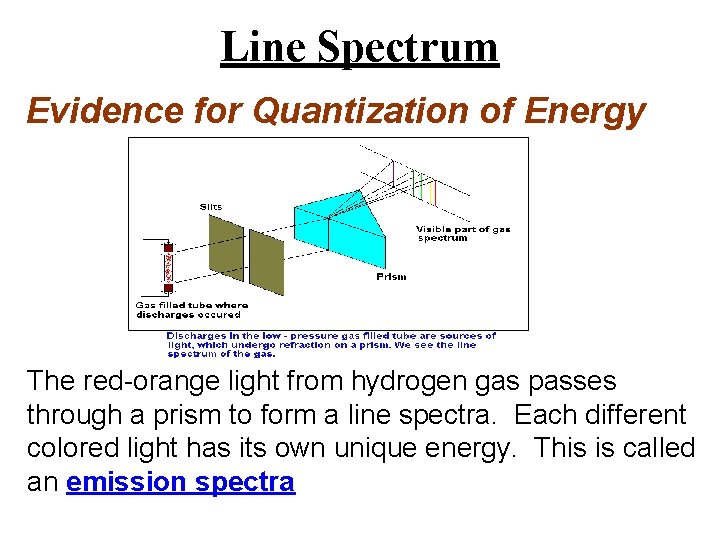
Line Spectrum Evidence for Quantization of Energy The red-orange light from hydrogen gas passes through a prism to form a line spectra. Each different colored light has its own unique energy. This is called an emission spectra

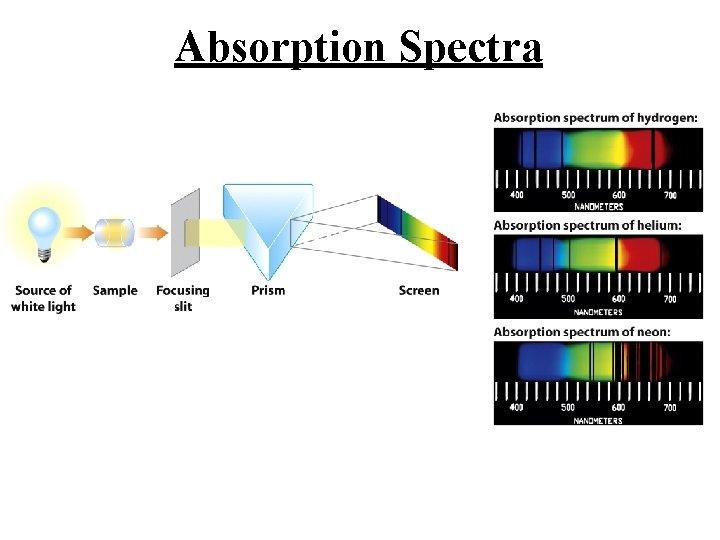
Absorption Spectra

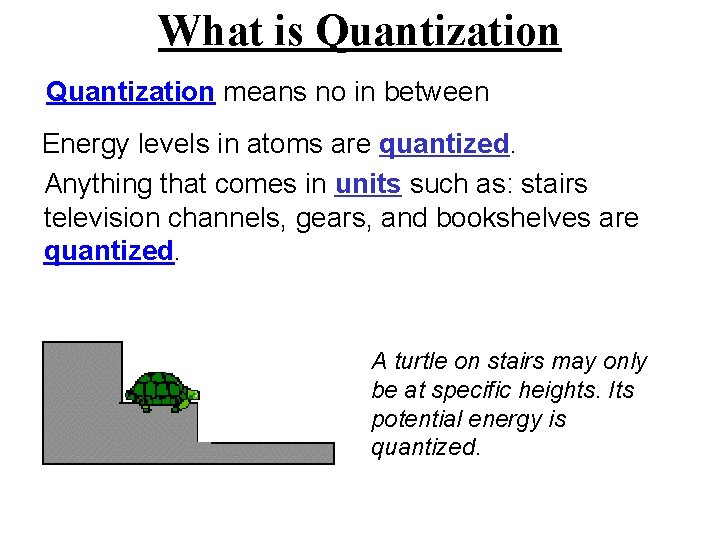
What is Quantization means no in between Energy levels in atoms are quantized. Anything that comes in units such as: stairs television channels, gears, and bookshelves are quantized. A turtle on stairs may only be at specific heights. Its potential energy is quantized.

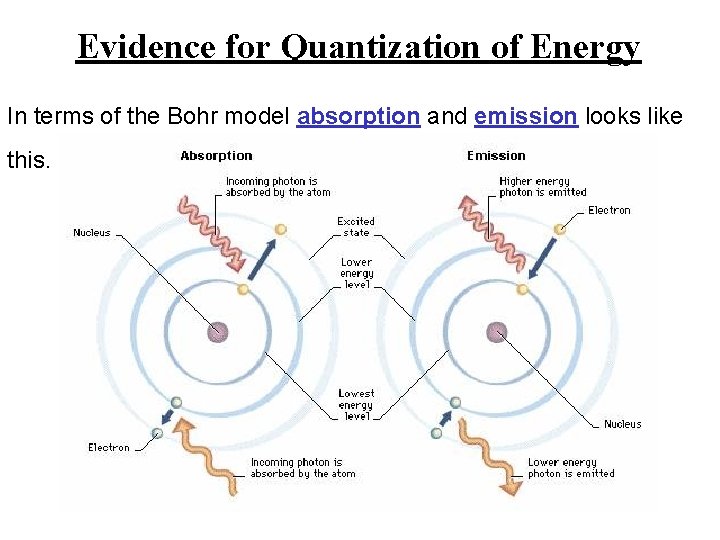
Evidence for Quantization of Energy In terms of the Bohr model absorption and emission looks like this.

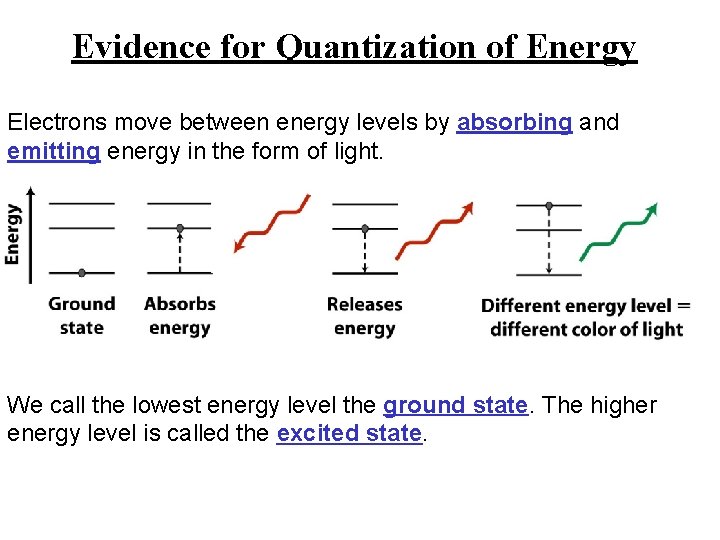
Evidence for Quantization of Energy Electrons move between energy levels by absorbing and emitting energy in the form of light. We call the lowest energy level the ground state. The higher energy level is called the excited state.

Evidence for Quantization of Energy The Bohr model works well for the hydrogen atom which has only one electron but performs poorly for more complex atoms. This led to the development of the current quantum mechanical model describing the arrangement of electrons in atoms. Unfortunately for us this model is more complex than that developed by Neils Bohr.

Quantum Numbers Each subshell is made up of one or more orbitals. An orbital is a volume of space where an electron is likely to be found. What is the orbital for books called? Fish? Cars? It is important not to confuse an orbit (a circular path on which an electron moves in the Bohr model) with an orbital. They are two very different things.

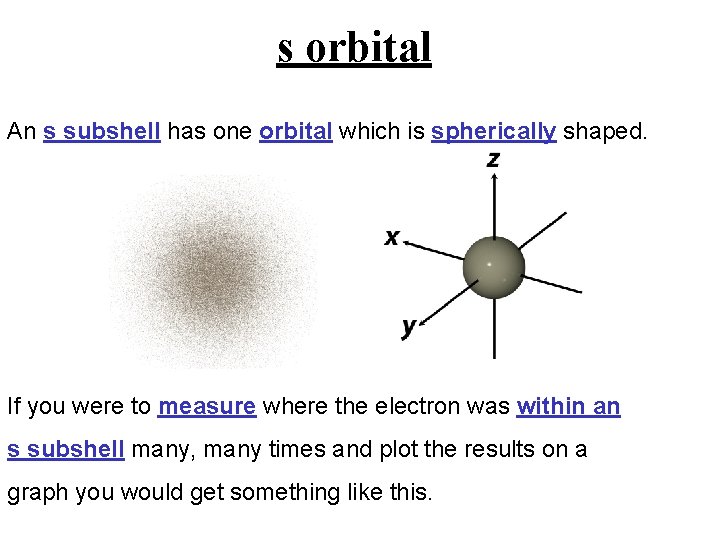
s orbital An s subshell has one orbital which is spherically shaped. If you were to measure where the electron was within an s subshell many, many times and plot the results on a graph you would get something like this.

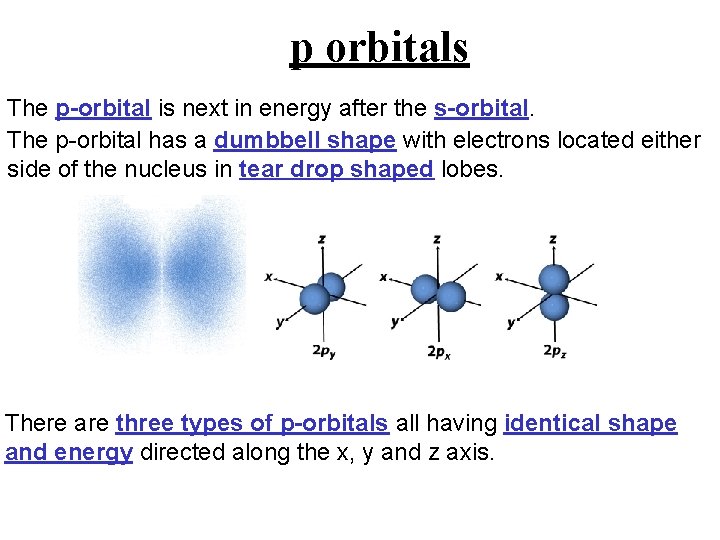
p orbitals The p-orbital is next in energy after the s-orbital. The p-orbital has a dumbbell shape with electrons located either side of the nucleus in tear drop shaped lobes. There are three types of p-orbitals all having identical shape and energy directed along the x, y and z axis.

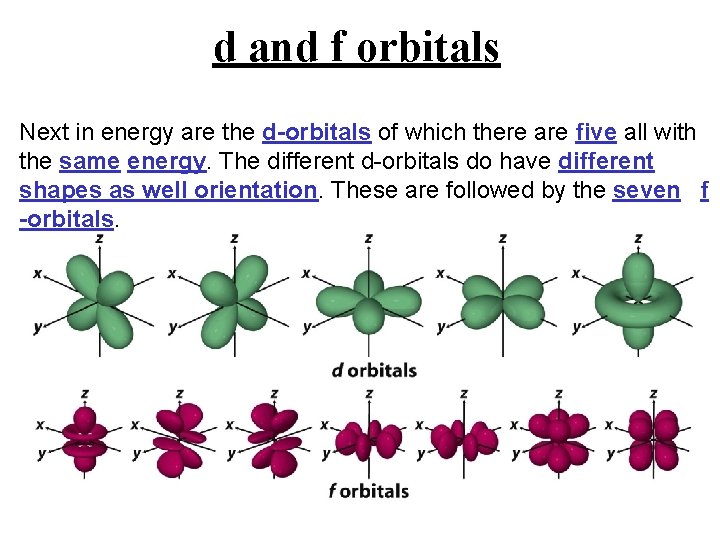
d and f orbitals Next in energy are the d-orbitals of which there are five all with the same energy. The different d-orbitals do have different shapes as well orientation. These are followed by the seven f -orbitals.

Energy Levels and Orbitals As mentioned earlier each shell has a number of subshells equal to its value for n. Therefore for: • n = 1 there will be one subshell 1 s • n = 2 there will be two subshells 2 s and 2 p • n = 3 there will be three subshells 3 s, 3 p and 3 d • n = 4 there will be four subshells 4 s, 4 p, 4 d and 4 f • n = 5 there will be four subshells 5 s, 5 p, 5 d and 5 f

Aufbau Principle The way in which electrons are organized into shells, subshells and orbitals in an atom is called the electronic configuration. The electronic configuration of an atom can be determined using the “Aufbau rule” also known as the “building up principle”. Aufbau comes from the German meaning construction although it was the Danish physicist Neils Bohr who came up with the idea !!

Aufbau Principle The Aufbau Principle states that: “The orbitals of lower energy are filled in first with the electrons and only then the orbitals of high energy are filled. ” What is the lowest energy orbital of an atom? 1 s orbital What is the third lowest energy orbital of an atom? 2 p orbital

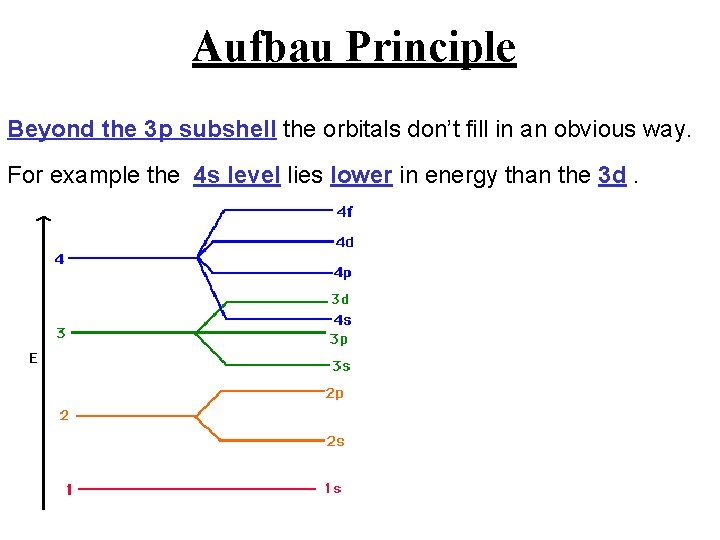
Aufbau Principle Beyond the 3 p subshell the orbitals don’t fill in an obvious way. For example the 4 s level lies lower in energy than the 3 d.

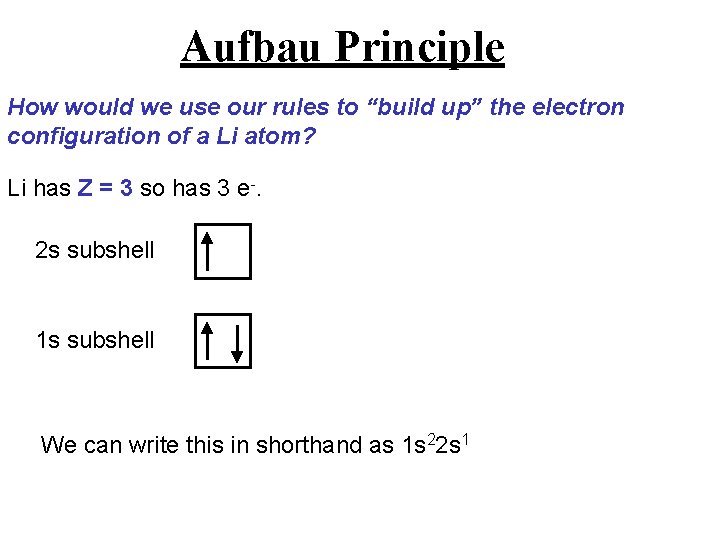
Aufbau Principle How would we use our rules to “build up” the electron configuration of a Li atom? Li has Z = 3 so has 3 e-. 2 s subshell 1 s subshell We can write this in shorthand as 1 s 22 s 1

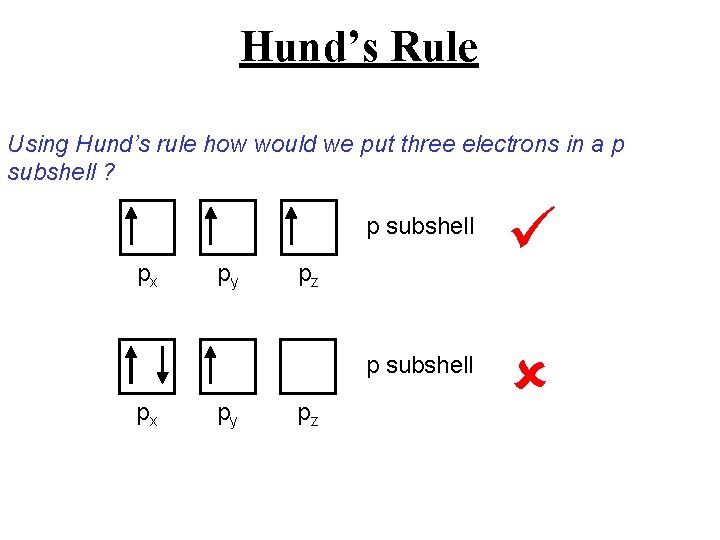
Hund’s Rule If there are multiple orbitals with the same energy how do we decide which orbital to put an electron? We use Hund’s rule which states: “Electrons fill degenerate orbitals one at a time before doubling up in the same orbital”

Hund’s Rule Using Hund’s rule how would we put three electrons in a p subshell ? px px py py p subshell pz pz

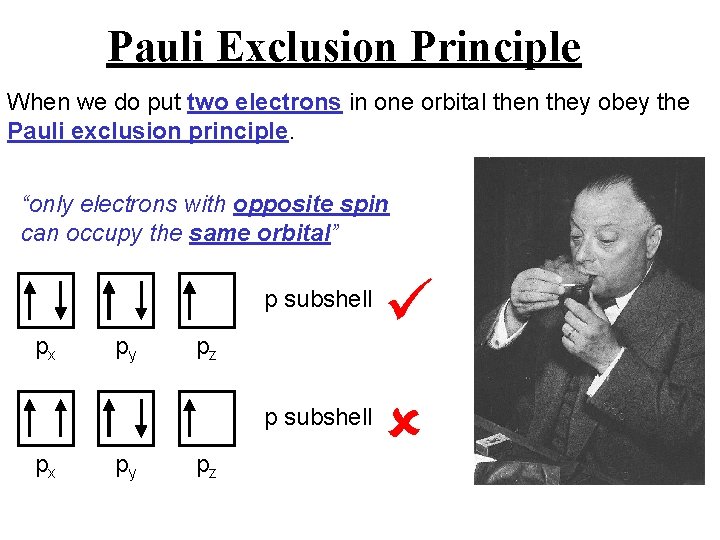
Pauli Exclusion Principle When we do put two electrons in one orbital then they obey the Pauli exclusion principle. “only electrons with opposite spin can occupy the same orbital” px px py py p subshell pz pz

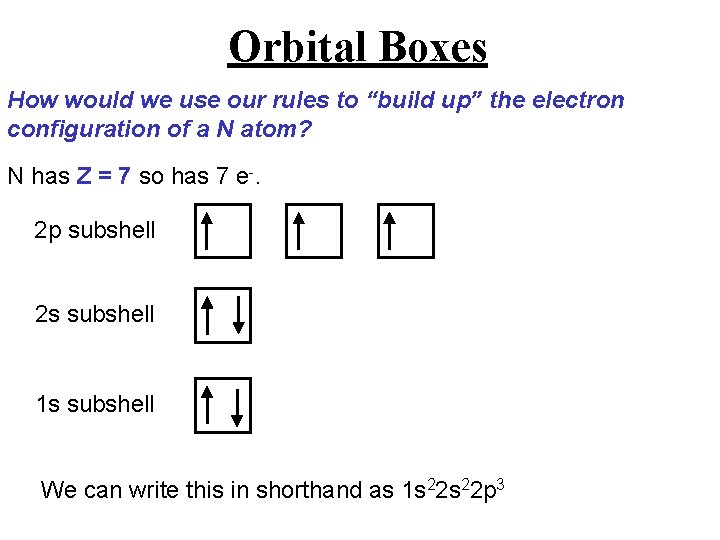
Orbital Boxes How would we use our rules to “build up” the electron configuration of a N atom? N has Z = 7 so has 7 e-. 2 p subshell 2 s subshell 1 s subshell We can write this in shorthand as 1 s 22 p 3

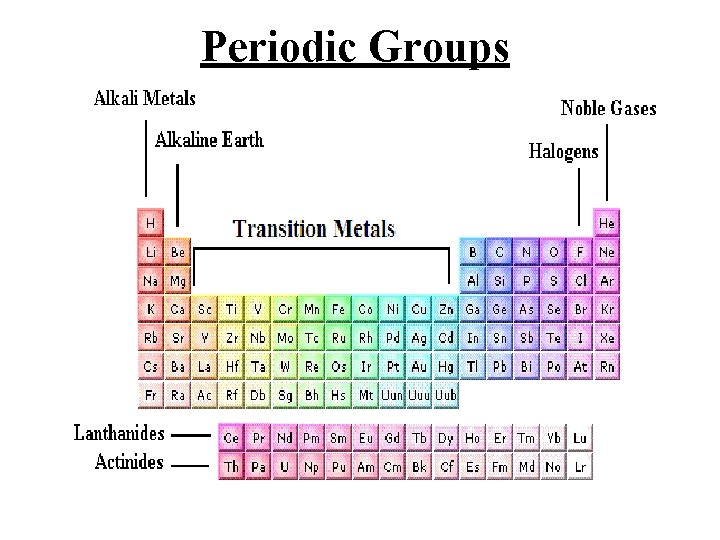
Valence Electrons The electrons in the highest energy shell of an atom are (those furthest from the nucleus) are called the valence electrons. These electrons are very important as when atoms interact with each other it is through their valence electrons.

Using the Periodic Table

How many electrons fill the following sublevels? a. The 3 rd level. b. The 4 th level. c. The 5 th level

How many electrons fill the following sublevels? a. The 3 rd level. 2(n)2 =2(3)2=18 b. The 4 th level. c. The 5 th level

How many electrons fill the following sublevels? a. The 3 rd level. 2(n)2 =2(3)2=18 b. The 4 th level. 2(n)2 =2(4)2=32 c. The 5 th level

How many electrons fill the following sublevels? a. The 3 rd level. 2(n)2 =2(3)2=18 b. The 4 th level. 2(n)2 =2(4)2=32 c. The 5 th level 2(n)2 =2(5)2=50

How many orbitals are in an atom with n=3?

How many orbitals are in an atom with n=3? Answer: n = 3 refers to the third energy level, which contains the s, p, and d sublevels. There is only one orbital in the s, three in the p, and five in the d sublevels; for a total of 9 orbitals.

Periodic Groups

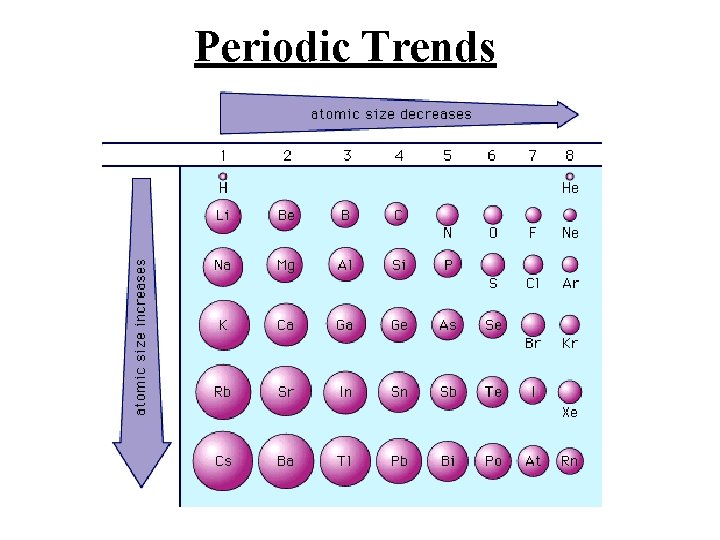
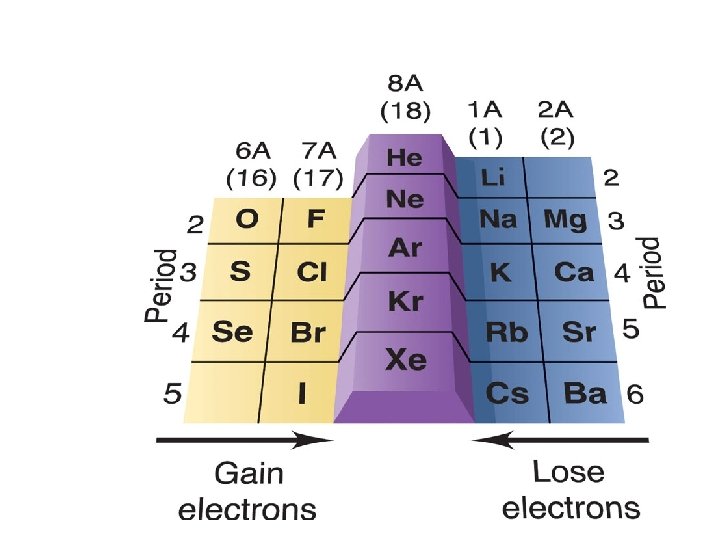
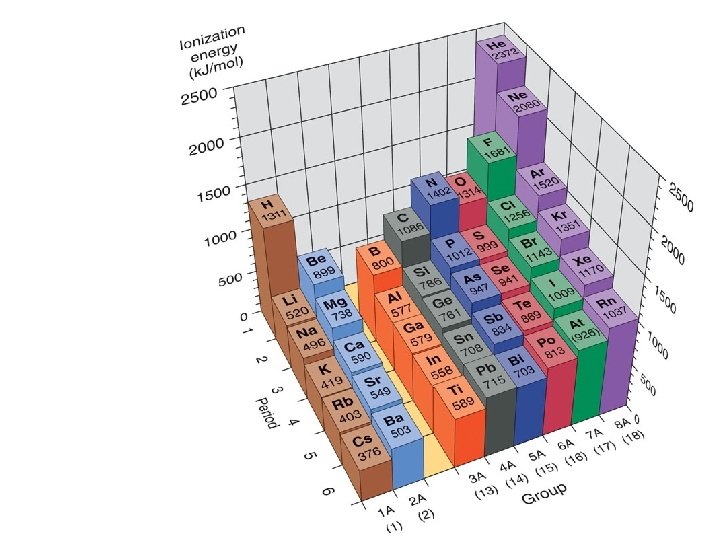
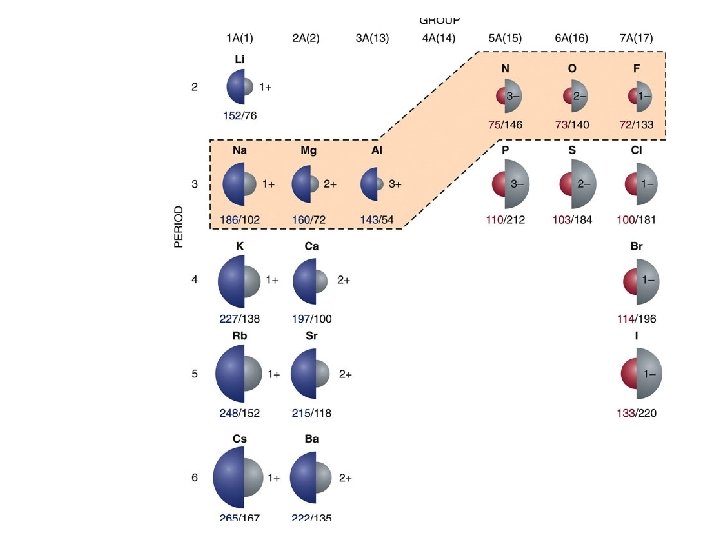
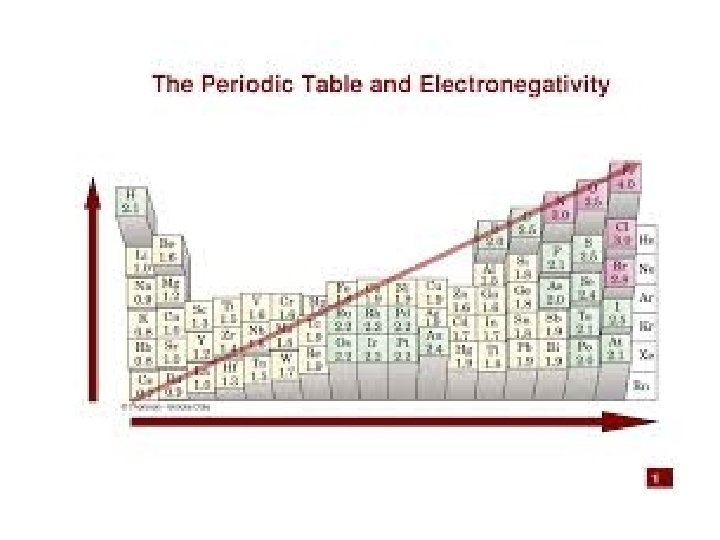
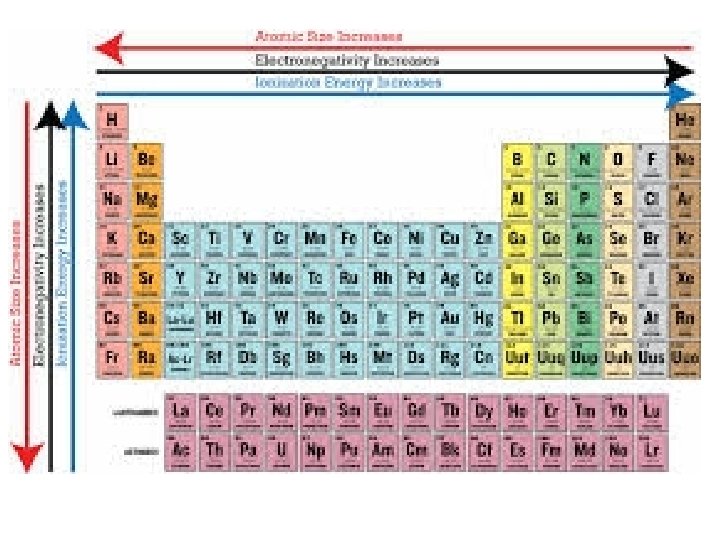
PERIODIC TRENDS 1. Atomic size a. Increases from top to bottom (gaining a new outer shell) b. Decreases left to right in a period (Increase of protons attracts electrons stronger, thus contracting the element. 2. Ionization Energy (relative ease of losing electrons) The larger the atom, the further electrons are from nucleus, and the less they are held by nucleus = lower ionization energy 3. Electronegativity ( “Desire” to take an electron. Closer to 8 valence electrons, small radius = higher electronegativity

Periodic Trends

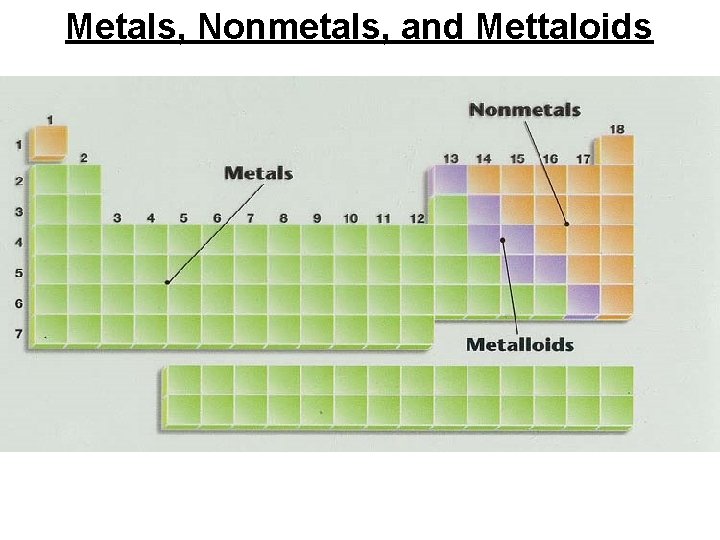
Metals, Nonmetals, and Mettaloids






Identify the element with the following electron configurations, also stating the group and period the where the element where found. a. 1 s 22 s 2 p 63 s 23 p 64 s 23 d 104 p 4 b. [Ar]3 s 23 d 7

Identify the element with the following electron configurations, also stating the group and period the where the element where found. a. 1 s 22 s 2 p 63 s 23 p 64 s 23 d 104 p 4 Se, Period 4, Group VI (6) b. [Ar]3 s 23 d 7

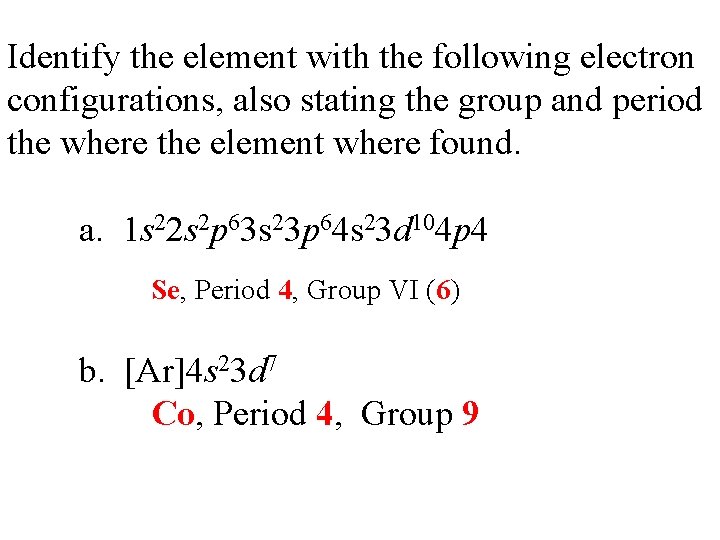
Identify the element with the following electron configurations, also stating the group and period the where the element where found. a. 1 s 22 s 2 p 63 s 23 p 64 s 23 d 104 p 4 Se, Period 4, Group VI (6) b. [Ar]4 s 23 d 7 Co, Period 4, Group 9

The End