Atomic Theory Periodic Trends Where Did it All



















- Slides: 48

Atomic Theory & Periodic Trends

Where Did it All Begin? • The word “atom” comes from the Greek word “atomos” which means indivisible. • The idea that all matter is made up of atoms was first proposed by the Greek philosopher Democritus in the 5 th century B. C.

Dalton’s Atomic Theory John Dalton (1766 -1844) proposed an atomic theory While this theory was not completely correct, it revolutionized how chemists looked at matter and brought about chemistry as we know it today instead of alchemy Thus, it’s an important landmark in the history of science.

Dalton’s Atomic Theory - Summary 1. matter is composed, indivisible particles (atoms) 2. all atoms of a particular element are identical 3. different elements have different atoms 4. atoms combine in certain whole-number ratios 5. In a chemical reaction, atoms are merely rearranged to form new compounds; they are not created, destroyed, or changed into atoms of any other elements.

Problems with Dalton’s Atomic Theory? 1. matter is composed, indivisible particles Atoms Can Be Divided, but only in a nuclear reaction 2. all atoms of a particular element are identical Does Not Account for Isotopes (atoms of the same element but a different mass due to a different number of neutrons)! 3. different elements have different atoms YES! 4. atoms combine in certain whole-number ratios YES! Called the Law of Definite Proportions 5. In a chemical reaction, atoms are merely rearranged to form new compounds; they are not created, destroyed, or changed into atoms of any other elements. Yes, except for nuclear reactions that can change atoms of one element to a different element

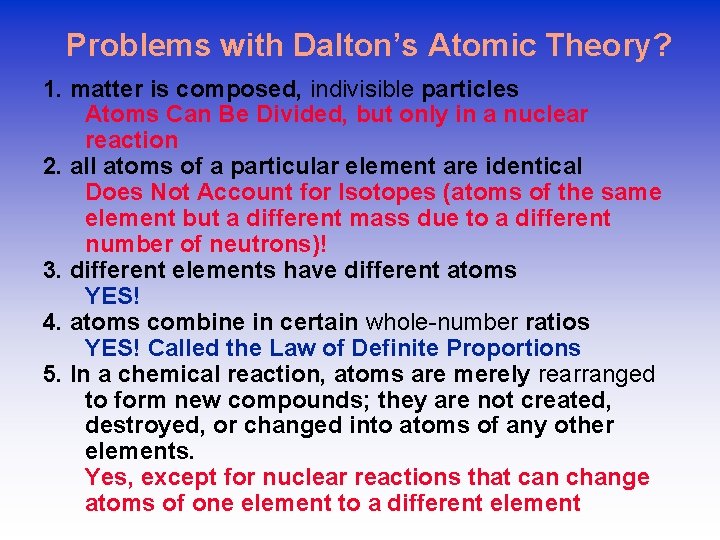
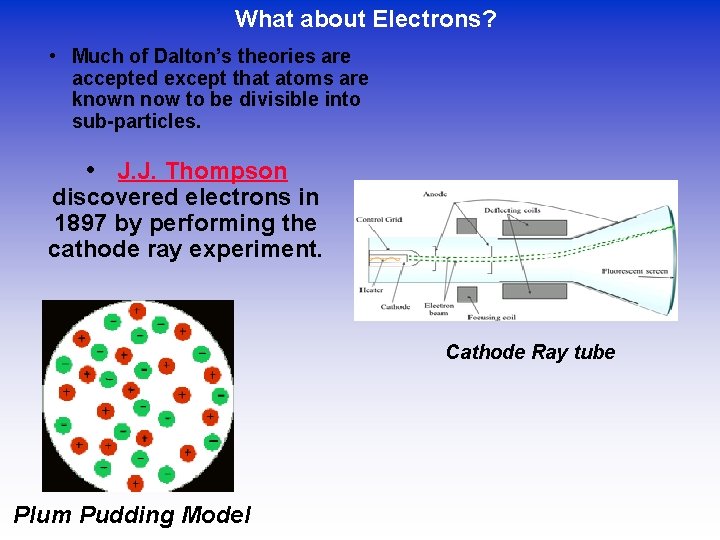
What about Electrons? • Much of Dalton’s theories are accepted except that atoms are known now to be divisible into sub-particles. • J. J. Thompson discovered electrons in 1897 by performing the cathode ray experiment. Cathode Ray tube Plum Pudding Model

• Physicist James Chadwick 1891 -1974 confirmed the existence of yet another subatomic particle: the neutron • Chadwick was awarded the Nobel Peace Prize for physics in 1935 and was also awarded the Hughes Model of the Royal Society in 1932. His discovery mad it possible to create with a greater mass than uranium in the laboratory. Because of Chadwick's discovery, "nuclear fission" was discovered.

Ernest Rutherford wondered how all these particles were put together Rutherford’s Gold Foil Experiment

The modern view of the atom was developed by Ernest Rutherford (1871 -1937).

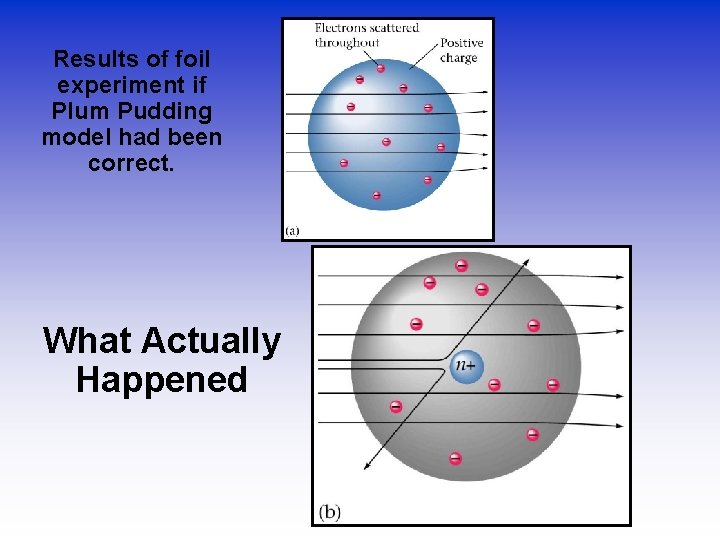
Results of foil experiment if Plum Pudding model had been correct. What Actually Happened

Niels Bohr 1925 • Niels Bohr applies quantum theory to Rutherford's atomic structure by assuming that electrons travel in stationary orbits defined by their angular momentum. • This led to the calculation of possible energy levels for these orbits and the postulation that the emission of light occurs when an electron moves into a lower energy orbit.

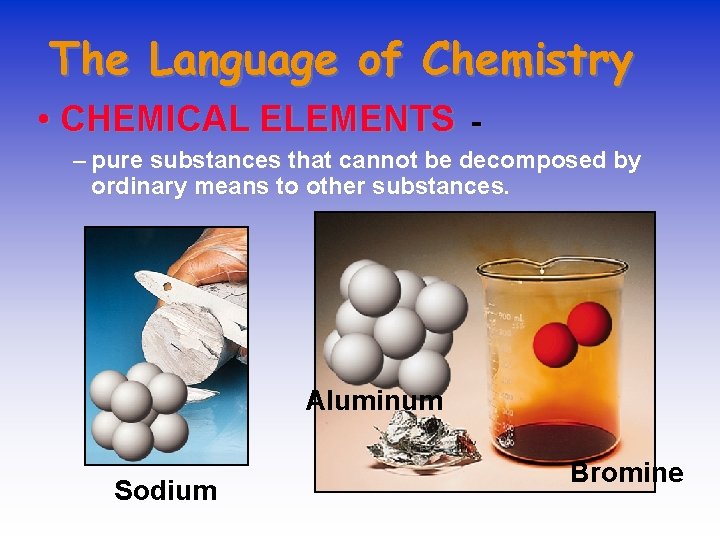
The Language of Chemistry • CHEMICAL ELEMENTS - – pure substances that cannot be decomposed by ordinary means to other substances. Aluminum Sodium Bromine

The Language of Chemistry • The elements, their names, and symbols are given on the PERIODIC TABLE • How many elements are there?

The Periodic Table Dmitri Mendeleev (1834 - 1907)

Glenn Seaborg (1912 -1999 ) • Discovered 8 new elements. • Only living person for whom an element was named.

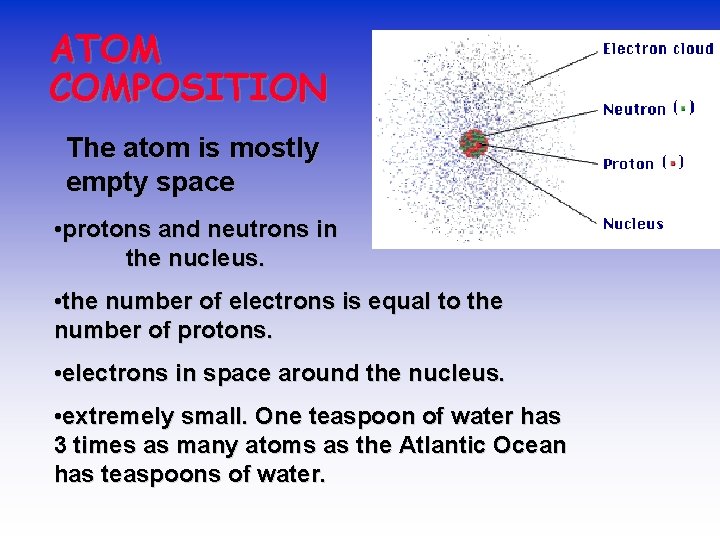
The Atom An atom consists of a • nucleus – (of protons and neutrons) • electrons in space about the nucleus. Electron cloud Nucleus

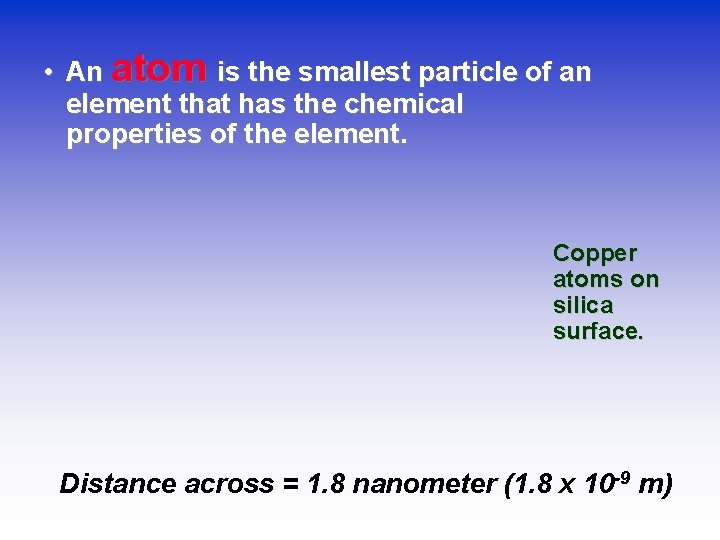
• An atom is the smallest particle of an element that has the chemical properties of the element. Copper atoms on silica surface. Distance across = 1. 8 nanometer (1. 8 x 10 -9 m)

ATOM COMPOSITION The atom is mostly empty space • protons and neutrons in the nucleus. • the number of electrons is equal to the number of protons. • electrons in space around the nucleus. • extremely small. One teaspoon of water has 3 times as many atoms as the Atlantic Ocean has teaspoons of water.

ATOMIC COMPOSITION • Protons (p+) – – – + electrical charge mass = 1. 672623 x 10 -24 g relative mass = 1. 007 atomic mass units (amu) but we can round to 1 • Electrons (e-) negative electrical charge – relative mass = 0. 0005 amu but we can round to 0 – • Neutrons (no) no electrical charge – mass = 1. 009 amu but we can round to 1 –

• But Why Does The Periodic Table end too quickly? Can’t we just add a proton to the end? ? ? • Let’s Watch!

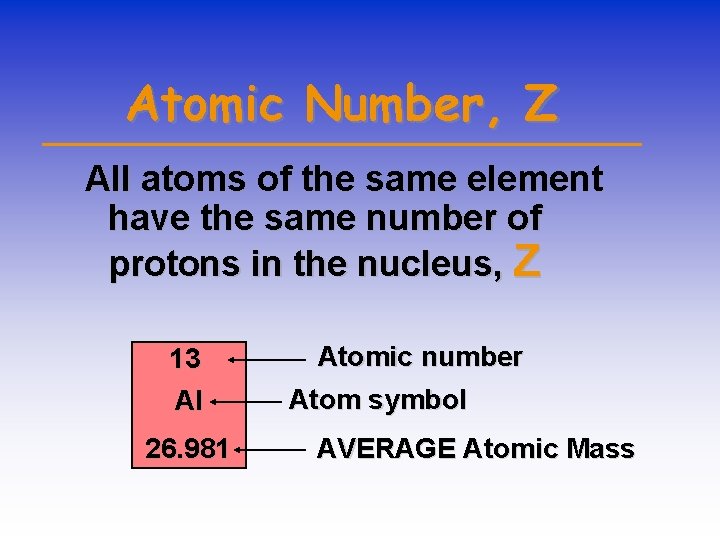
Atomic Number, Z All atoms of the same element have the same number of protons in the nucleus, Z 13 Al 26. 981 Atomic number Atom symbol AVERAGE Atomic Mass

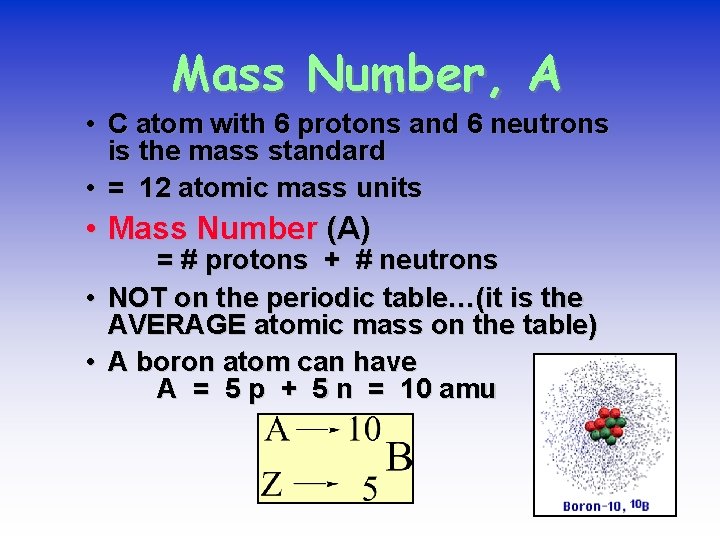
Mass Number, A • C atom with 6 protons and 6 neutrons is the mass standard • = 12 atomic mass units • Mass Number (A) • • = # protons + # neutrons NOT on the periodic table…(it is the AVERAGE atomic mass on the table) A boron atom can have A = 5 p + 5 n = 10 amu

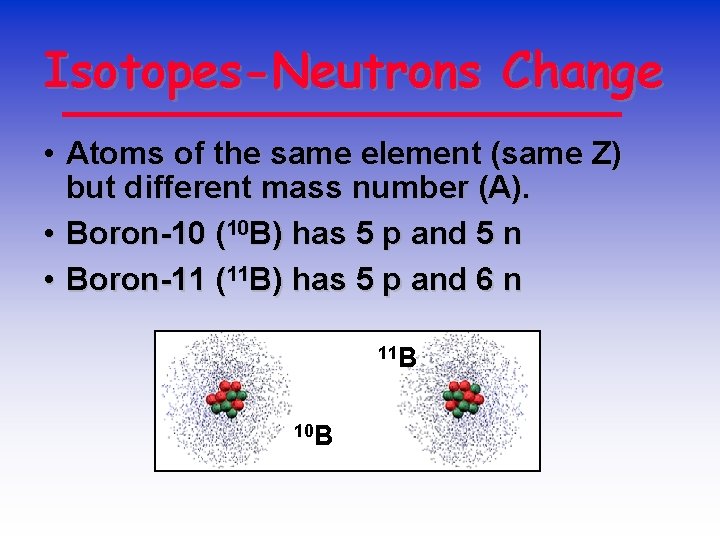
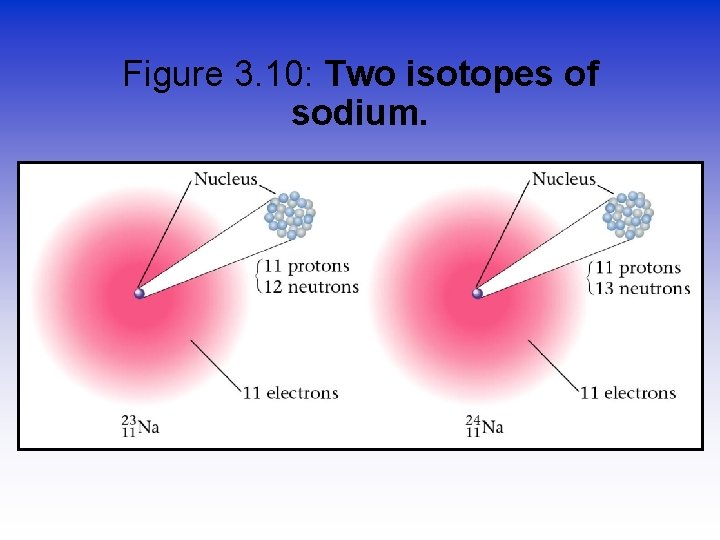
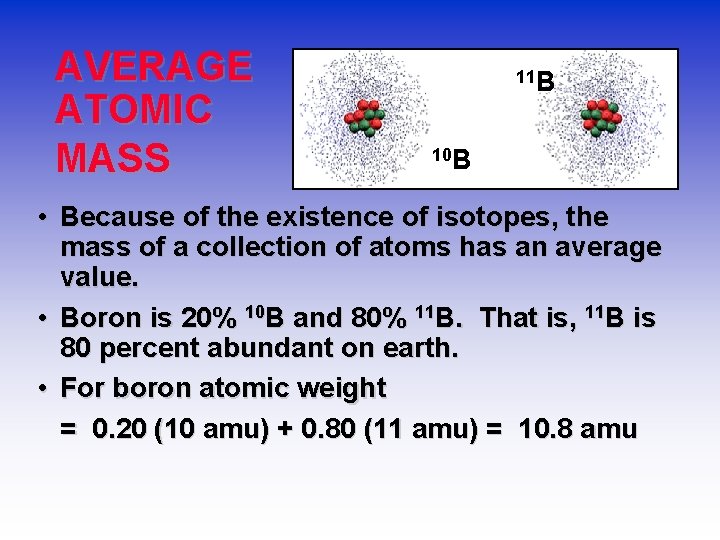
Isotopes-Neutrons Change • Atoms of the same element (same Z) but different mass number (A). • Boron-10 (10 B) has 5 p and 5 n • Boron-11 (11 B) has 5 p and 6 n 11 B 10 B

Figure 3. 10: Two isotopes of sodium.

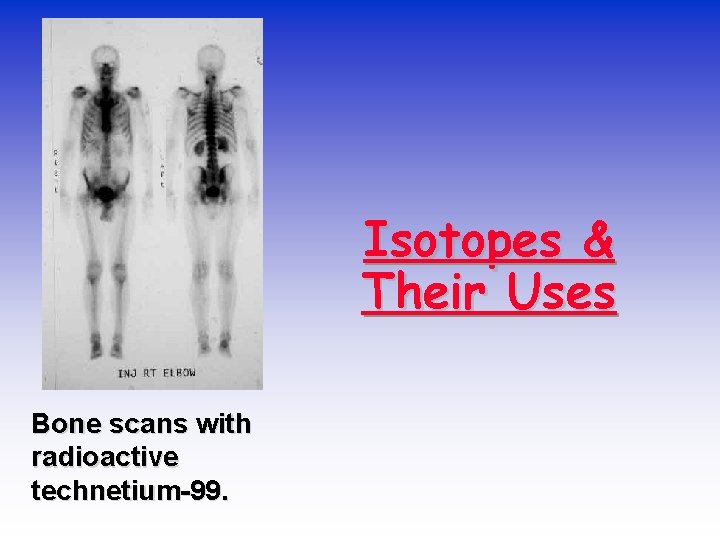
Isotopes & Their Uses Bone scans with radioactive technetium-99.

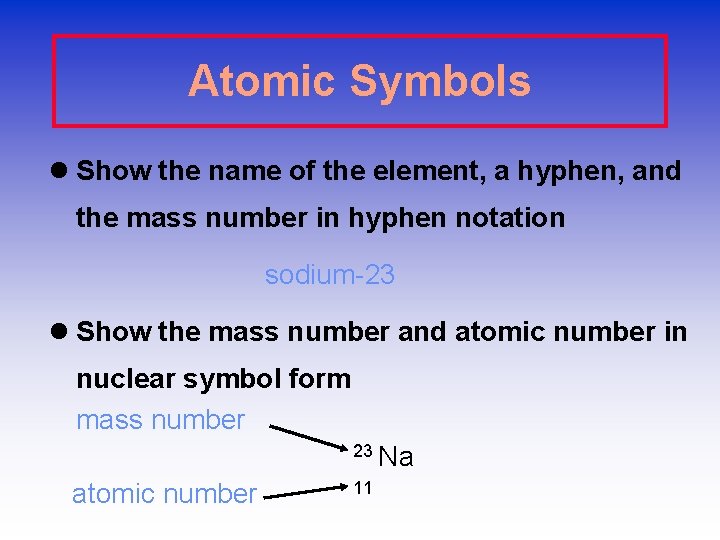
Atomic Symbols l Show the name of the element, a hyphen, and the mass number in hyphen notation sodium-23 l Show the mass number and atomic number in nuclear symbol form mass number 23 Na atomic number 11

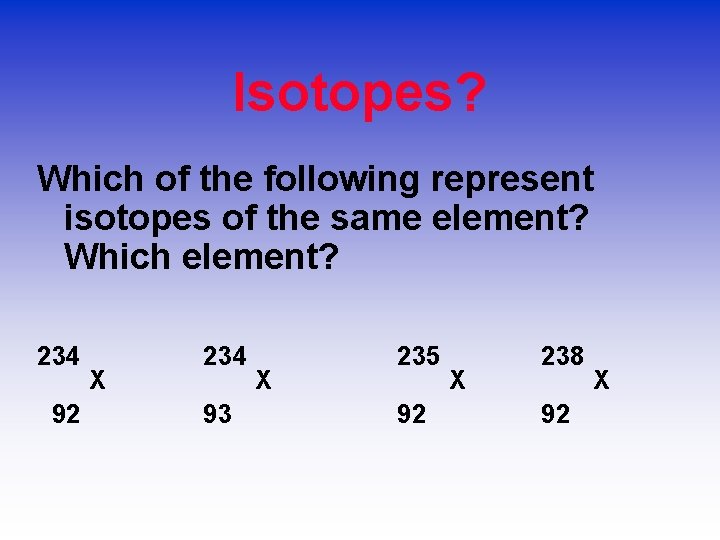
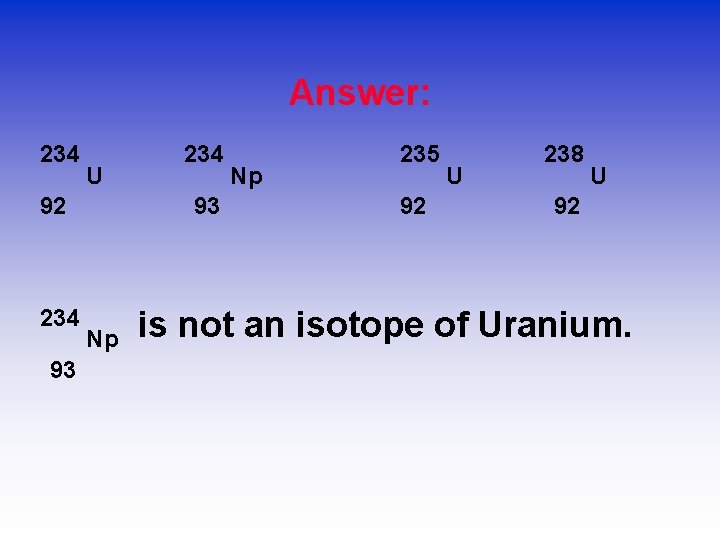
Isotopes? Which of the following represent isotopes of the same element? Which element? 234 92 X 234 93 X 235 92 X 238 92 X

Answer: 234 U 92 234 93 Np Np 235 92 U 238 U 92 is not an isotope of Uranium.

Counting Protons, Neutrons, and Electrons • Protons: Atomic Number (from periodic table) • Neutrons: Mass Number minus the number of protons (mass number is protons and neutrons because the mass of electrons is negligible) • Electrons: – If it’s an atom, the protons and electrons must be the SAME so that it is has a net charge of zero (equal numbers of + and -) – If it does NOT have an equal number of electrons, it is not an atom, it is an ION. For each negative charge, add an extra electron. For each positive charge, subtract an electron (Don’t add a proton!!! That changes the element!)

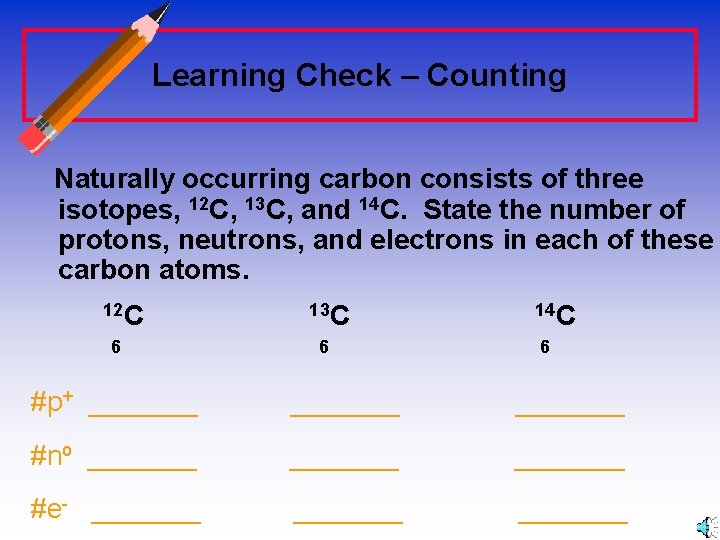
Learning Check – Counting Naturally occurring carbon consists of three isotopes, 12 C, 13 C, and 14 C. State the number of protons, neutrons, and electrons in each of these carbon atoms. 12 C 6 13 C 6 14 C 6 #p+ _______ #no _______ #e- _______

Answers 12 C 6 13 C 14 C 6 6 #p+ 6 6 6 #no 6 7 8 #e- 6 6 6

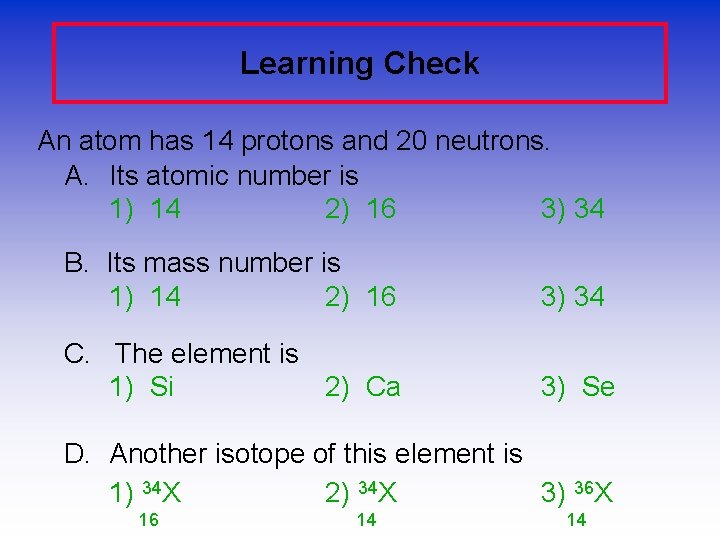
Learning Check An atom has 14 protons and 20 neutrons. A. Its atomic number is 1) 14 2) 16 3) 34 B. Its mass number is 1) 14 2) 16 3) 34 C. The element is 1) Si 2) Ca 3) Se D. Another isotope of this element is 1) 34 X 2) 34 X 3) 36 X 16 14 14

Solution An atom has 14 protons and 20 neutrons. A. It has atomic number 1) 14 B. It has a mass number of 3) 34 C. The element is 1) Si D. Another isotope of this element would be 3) 36 X 14

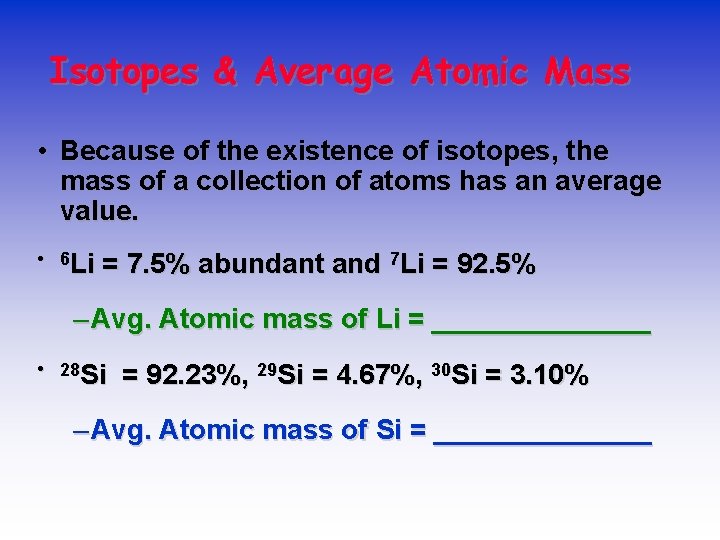
AVERAGE ATOMIC MASS 11 B 10 B • Because of the existence of isotopes, the mass of a collection of atoms has an average value. • Boron is 20% 10 B and 80% 11 B. That is, 11 B is 80 percent abundant on earth. • For boron atomic weight = 0. 20 (10 amu) + 0. 80 (11 amu) = 10. 8 amu

Isotopes & Average Atomic Mass • Because of the existence of isotopes, the mass of a collection of atoms has an average value. • 6 Li = 7. 5% abundant and 7 Li = 92. 5% – Avg. Atomic mass of Li = _______ • 28 Si = 92. 23%, 29 Si = 4. 67%, 30 Si = 3. 10% – Avg. Atomic mass of Si = _______

Nuclear Chemistry

NUCLEAR CHEMISTRY • In 1896 - Antoine Becquerel discovered radioactivity. • He accidentally left uranium ore on top of photographic plates. They became fogged from exposure to the radiation. – Becquerel had two graduate students: Marie and Pierre Curie.

• Radioactivity- the property by which uranium gives off rays. • Radiation-penetrating rays emitted by a radioactive source. – In 1903, the Curies and Becquerel won Nobel prizes for this discovery. – Marie Curie is still the only woman to win the Nobel prize in Chemistry and Physics

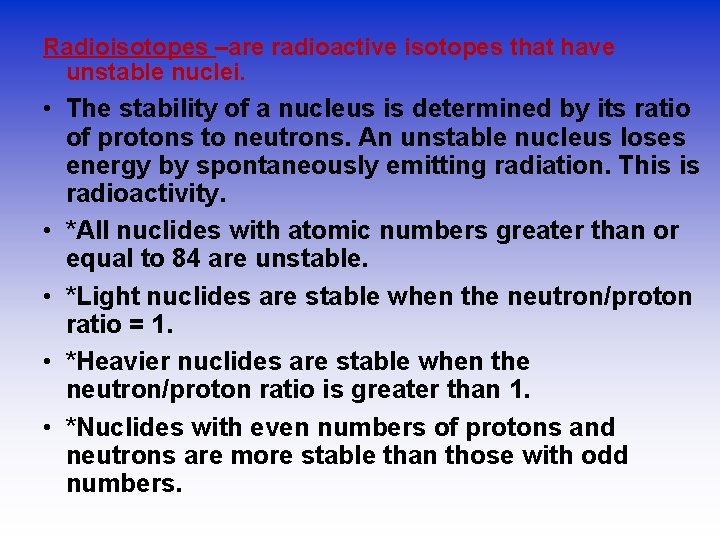
Radioisotopes –are radioactive isotopes that have unstable nuclei. • The stability of a nucleus is determined by its ratio of protons to neutrons. An unstable nucleus loses energy by spontaneously emitting radiation. This is radioactivity. • *All nuclides with atomic numbers greater than or equal to 84 are unstable. • *Light nuclides are stable when the neutron/proton ratio = 1. • *Heavier nuclides are stable when the neutron/proton ratio is greater than 1. • *Nuclides with even numbers of protons and neutrons are more stable than those with odd numbers.

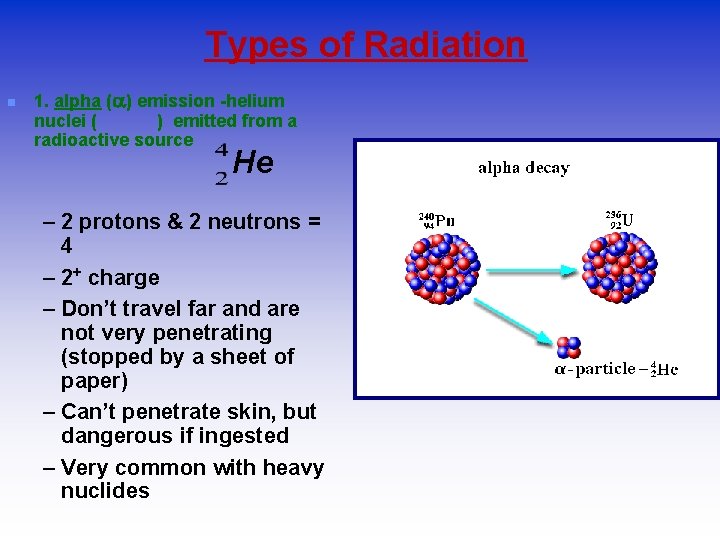
Types of Radiation n 1. alpha ( ) emission -helium nuclei ( ) emitted from a radioactive source He – 2 protons & 2 neutrons = 4 – 2+ charge – Don’t travel far and are not very penetrating (stopped by a sheet of paper) – Can’t penetrate skin, but dangerous if ingested – Very common with heavy nuclides

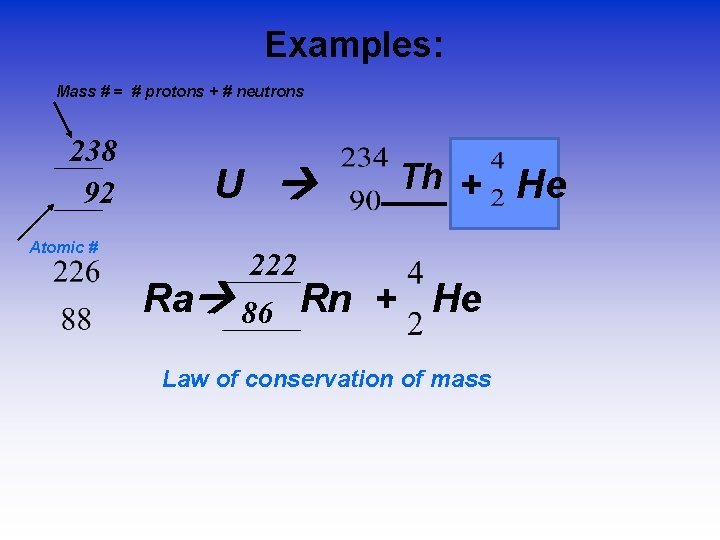
Examples: Mass # = # protons + # neutrons 238 92 Atomic # U 222 Ra 86 Rn Th + He ___ + He Law of conservation of mass

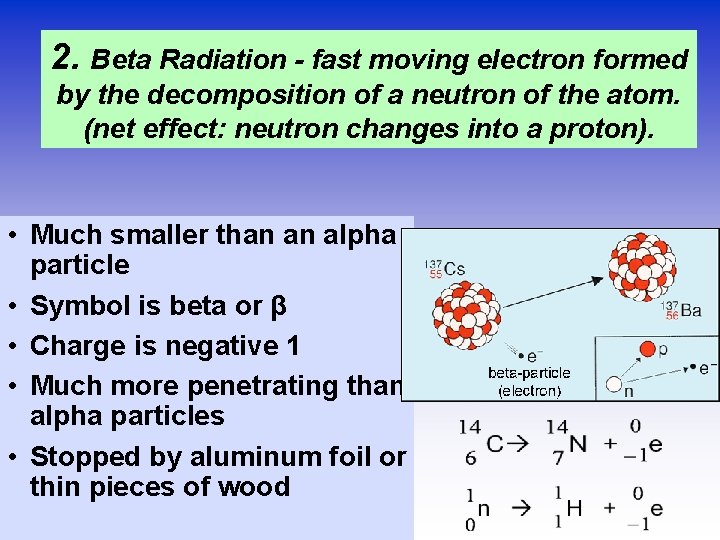
2. Beta Radiation - fast moving electron formed by the decomposition of a neutron of the atom. (net effect: neutron changes into a proton). • Much smaller than an alpha particle • Symbol is beta or β • Charge is negative 1 • Much more penetrating than alpha particles • Stopped by aluminum foil or thin pieces of wood

3. Gamma emission – electromagnetic radiation (high energy) emitted from a nucleus as it changes from an excited state to a ground energy state. • Often emitted along with alpha or beta radiation • Symbol is gamma or γ • Has no charge and no mass • High energy photon • Extremely penetrating, very dangerous • Stopped somewhat by several feet of concrete or several inches of lead.

Question • When nuclear power was first developed, people thought they could protect themselves from a nuclear blast by hiding in their basements. Was this a correct assumption?

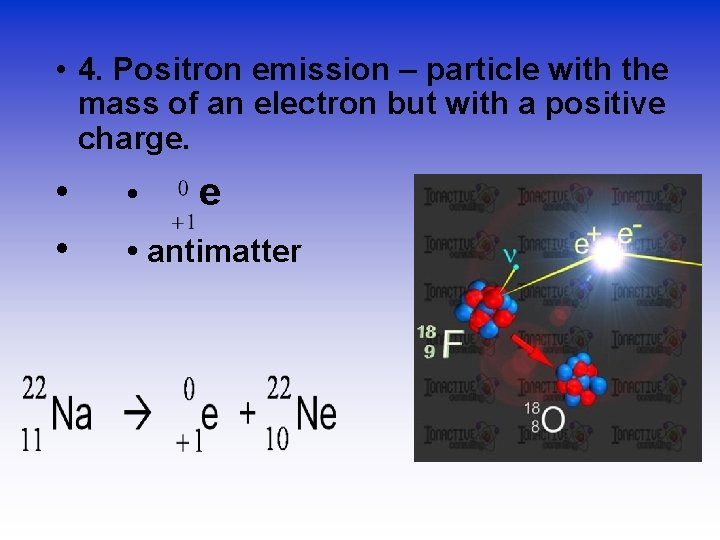
• 4. Positron emission – particle with the mass of an electron but with a positive charge. • • • e • antimatter

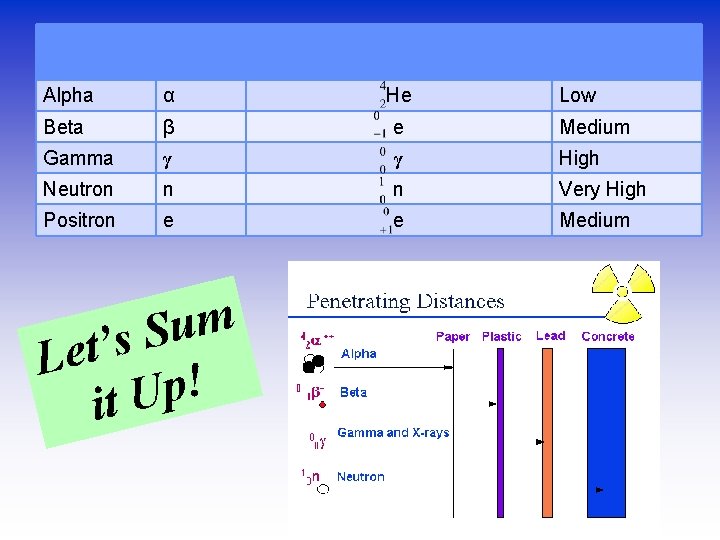
Type of radiation Symbol with mass and charge Penetration Alpha α He Beta β e Medium Gamma γ γ High Neutron n n Very High Positron e e Medium m u S s ’ t e L ! p U it Low

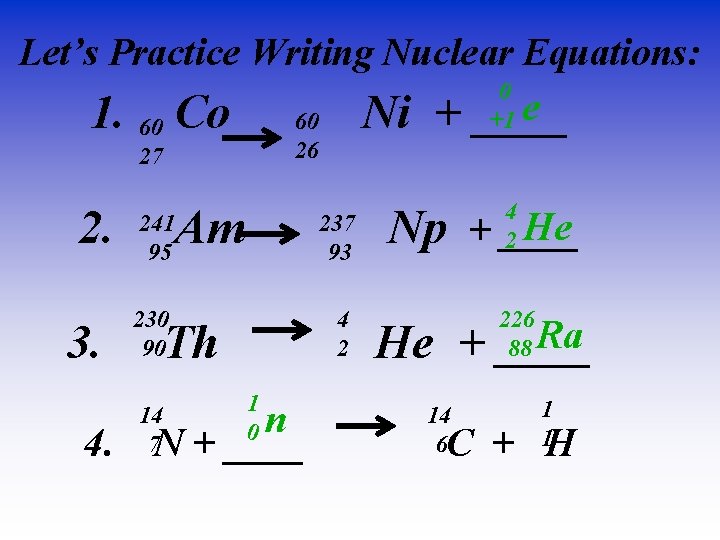
Let’s Practice Writing Nuclear Equations: 0 +1 e 1. 60 Co 60 Ni + ____ 26 27 2. 3. Am 241 95 237 93 230 90 4 2 Th 1 0 n 4. N + ____ 14 7 4 2 He Np + ____ 226 88 Ra He + ____ 14 6 1 1 C + H

This is the way that heavy elements are created that are added onto the periodic table. How far will it go? Only time and our technology can tell! • Nuclear Transformation (Transmutation)- changing one element into another. – bombarding with alpha particles N + He – Bombarding with neutrons U + O + n U H