Atomic Theory Nuclear Decay Review of Changes in






- Slides: 14

Atomic Theory Nuclear Decay

Review of Changes in Nature • Remember from Unit 1 that there are three types of changes in nature. • They are Physical, Chemical, and Nuclear

Physical Changes • These are changes in the size, shape, appearance, etc – but with no change in the chemical identity of what you have. • You still have the same stuff… • Examples of these changes are cutting paper with scissors, demolishing a building, boiling water, breaking a piece of glass.

Chemical Changes • These are changes in size, shape, appearance, etc – but here you do see the formation of new substances that were not previously in the system. • But – all of the atoms retain their chemical identity – they are simply connected to different atoms than they were before the change. • Examples here are burning paper, explosions, mixing two solutions to form a precipitate, milk going sour, biological decay, leaves changing color, and metals being consumed by acids.

Now for Nuclear Changes • These are changes in • The end result here is the internal structure of that you get new atoms themselves. of different elements. • The numbers of protons and neutrons are changed.

Alpha Decay • This is the first of the nuclear change processes. • In this scenario, an unstable nucleus emits (means it “spits out”) an alpha particle. • An alpha particle is simply a helium – 4 nucleus. It has 2 protons and 2 neutrons. • We represent it like this: 4 He 2

Nuclear Equation • This is the chemist’s method of representing a nuclear change in a format that looks like a chemical equation. • Example: “Write the nuclear equation for the alpha decay of Uranium-235”.

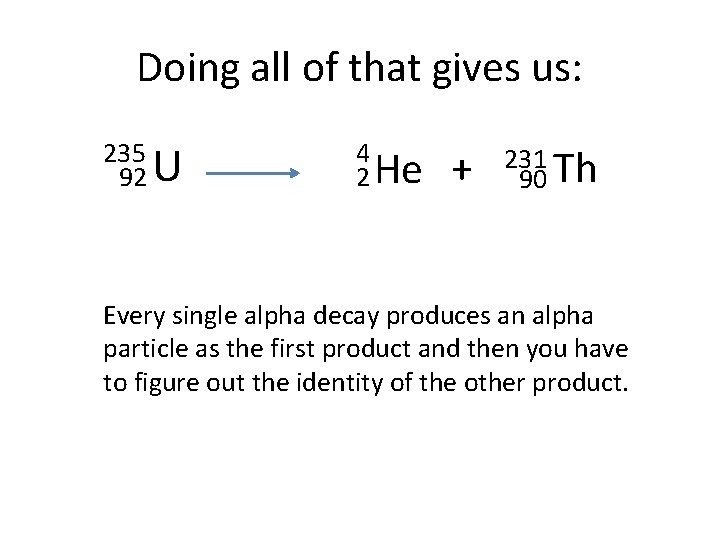
How to do this… • The starting point is given – it is the Uranium 235. • Since the example indicates that this is an alpha decay, the 1 st product in the equation will be an alpha particle. • The only task will be to determine the identity of the other product. • So…writing what we know so far…

Gives this… 235 92 U 4 2 He + ? To determine the identity of the other product, we have to subtract the 4 from the 235 to get the new mass number and then subtract the 2 from the 92 to find the new atomic number. Once we have the new atomic number, we can use the periodic chart to find its atomic symbol.

Doing all of that gives us: 235 92 U 4 2 He + 231 Th 90 Every single alpha decay produces an alpha particle as the first product and then you have to figure out the identity of the other product.

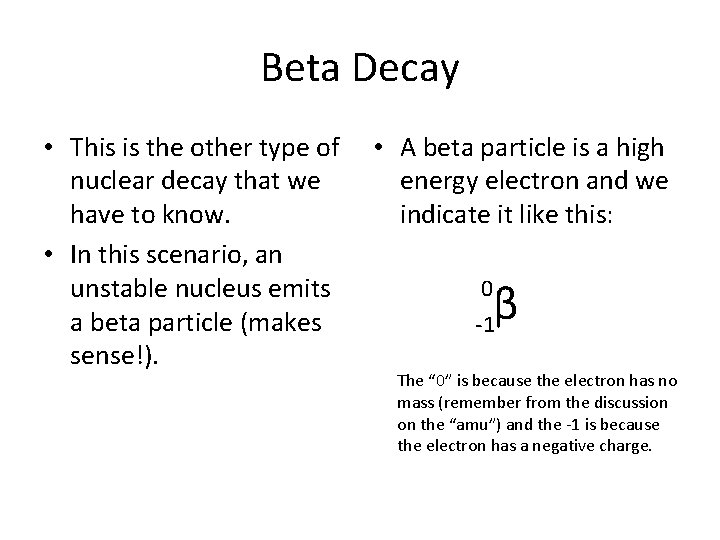
Beta Decay • This is the other type of nuclear decay that we have to know. • In this scenario, an unstable nucleus emits a beta particle (makes sense!). • A beta particle is a high energy electron and we indicate it like this: β -1 0 The “ 0” is because the electron has no mass (remember from the discussion on the “amu”) and the -1 is because the electron has a negative charge.

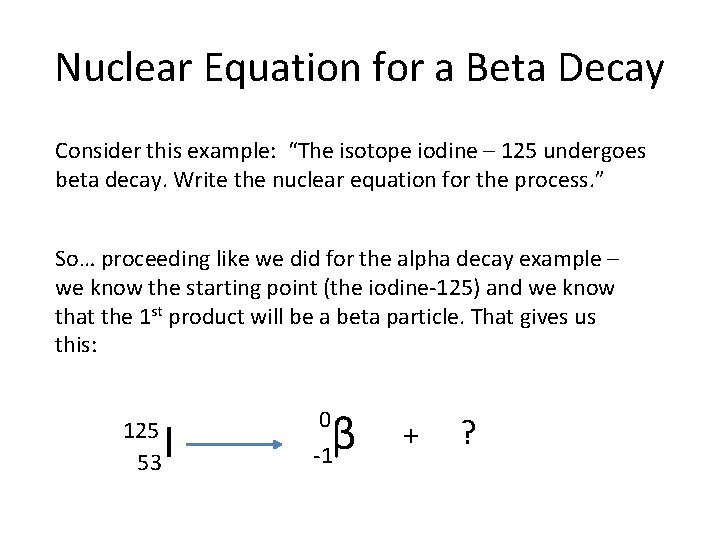
Nuclear Equation for a Beta Decay Consider this example: “The isotope iodine – 125 undergoes beta decay. Write the nuclear equation for the process. ” So… proceeding like we did for the alpha decay example – we know the starting point (the iodine-125) and we know that the 1 st product will be a beta particle. That gives us this: 125 53 I β -1 0 + ?

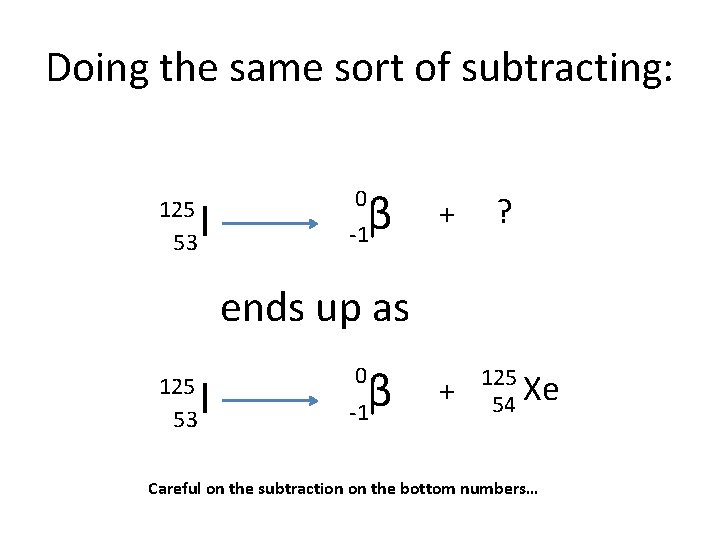
Doing the same sort of subtracting: 125 53 I β -1 0 + ? ends up as 125 53 I β -1 0 + 125 Xe 54 Careful on the subtraction on the bottom numbers…

Your assignment: • Complete the • Use the Modern worksheet that will be Chemistry book to provided by the teacher. determine which of the following nuclear events • Use the periodic chart has the greatest to determine the atomic “penetrating strength” symbols that go with (will go through the atomic numbers. most stuff). – Alpha particle, Beta particle, or Gamma radiation.