Atomic Theory and Structure Chapters 4 5 Atomic




















- Slides: 50

Atomic Theory and Structure Chapters 4 -5

Atomic Theories l Democritus ~ 400 BC l l believed that atoms were indivisible and indestructible Dalton ~ 1800’s l l Developed through experiments First Atomic Model

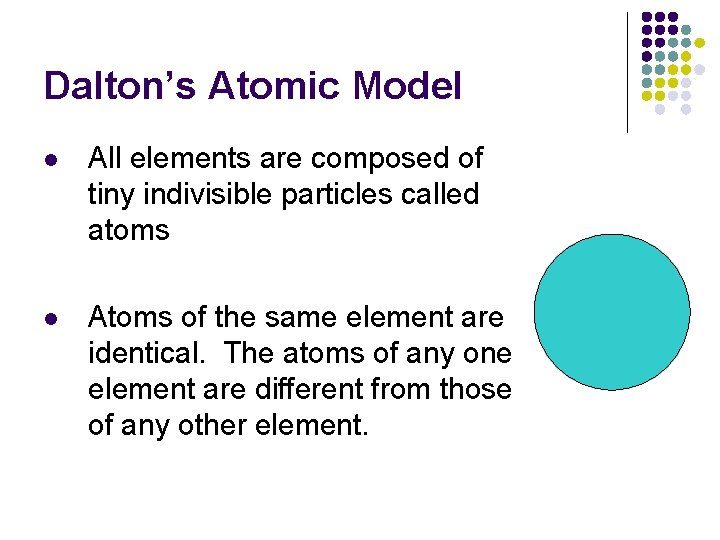
Dalton’s Atomic Model l All elements are composed of tiny indivisible particles called atoms l Atoms of the same element are identical. The atoms of any one element are different from those of any other element.

Dalton’s Atomic Model (cont) l Atoms of different elements can physically mix together or can chemically combine in simple whole-number ratios to form compounds. l Chemical reactions occur when atoms are separated, joined, or rearranged. Atoms of one element, however, are never changed into atoms of another element as a result of a chemical reaction.

Discovery of Electron l 1897 – JJ Thomson, using cathode ray tube, discovered negatively charged particles called electrons l 1909 – Robert Millikan - Oil Drop Experiment l Determined charge on an electron.

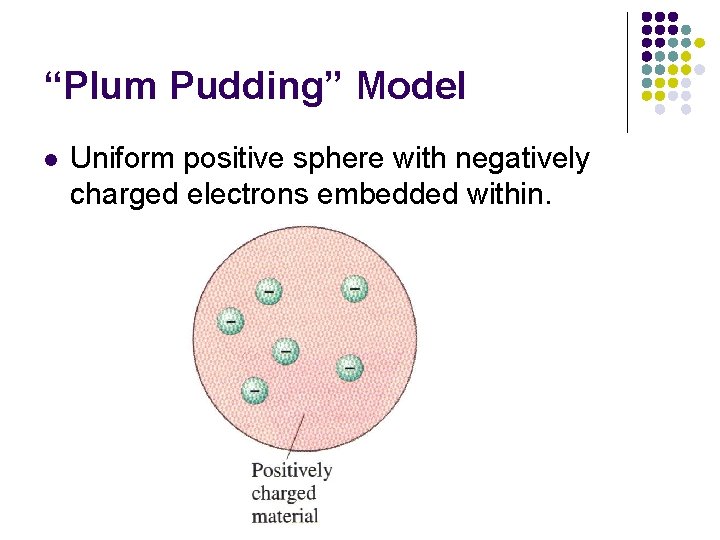
“Plum Pudding” Model l Uniform positive sphere with negatively charged electrons embedded within.

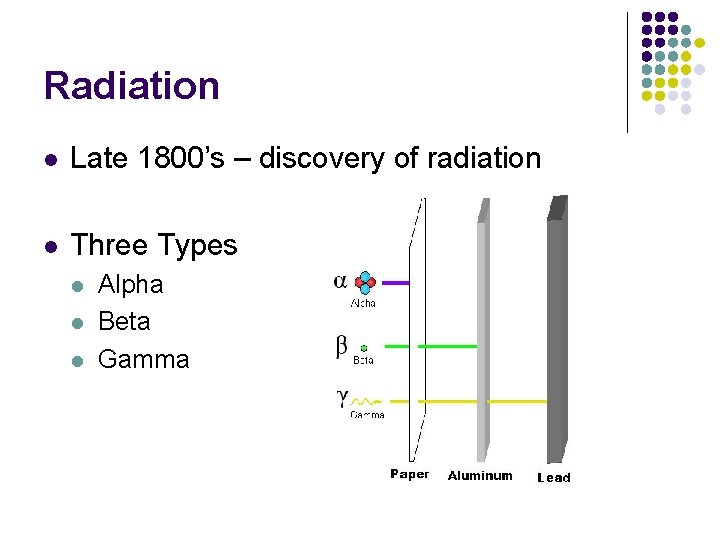
Radiation l Late 1800’s – discovery of radiation l Three Types l l l Alpha Beta Gamma

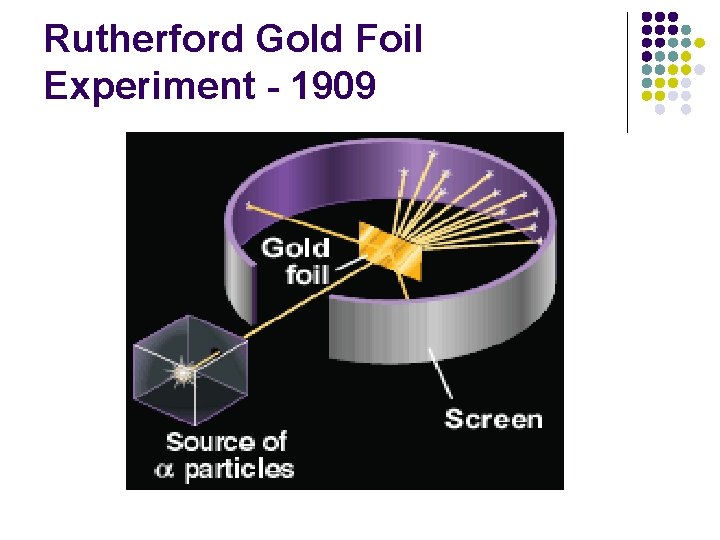
Rutherford Gold Foil Experiment - 1909 l l l Shot alpha particles at gold foil Most went through foil with little or no deflection. Some were deflected at large angle and some straight back. l A. K. A. Geiger Marsden Experiment

Rutherford Gold Foil Experiment - 1909

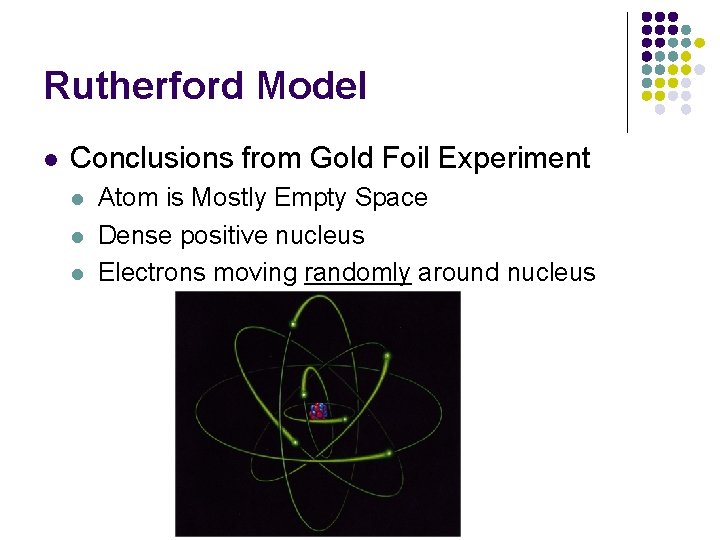
Rutherford Model l Conclusions from Gold Foil Experiment l l l Atom is Mostly Empty Space Dense positive nucleus Electrons moving randomly around nucleus

Subatomic Particles l Electron l l l Discovered in 1897 by JJ Thomson Negative charge (-1) Mass = 9. 109389*10 -28 g Approx mass ~ 0 Found outside of nucleus

Subatomic Particles l Proton l l l Discovered in 1919 by Rutherford Positive charge (+1) Mass = 1. 672623*10 -24 g Approx mass ~ 1 atomic mass unit (u) Found inside nucleus

Subatomic Particles l Neutron l l l Discovered in 1932 by James Chadwick No charge (0) Mass = 1. 6749286*10 -24 g Approx mass ~ 1 atomic mass unit (u) Just slightly larger than a proton Found inside nucleus

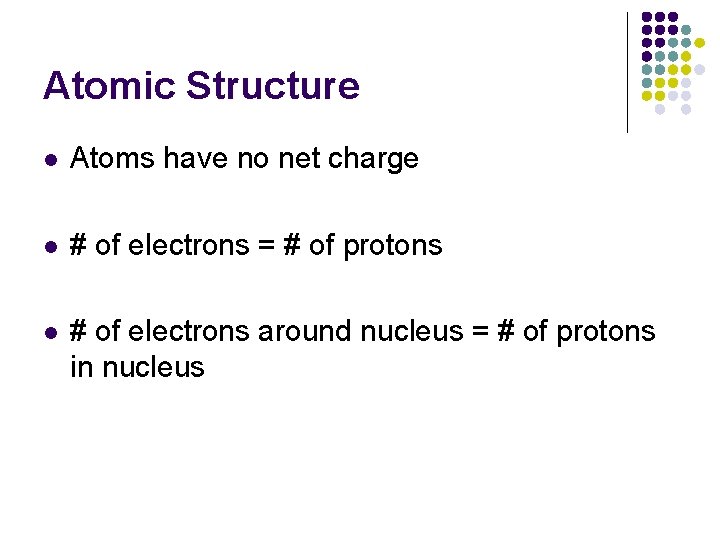
Atomic Structure l Atoms have no net charge l # of electrons = # of protons l # of electrons around nucleus = # of protons in nucleus

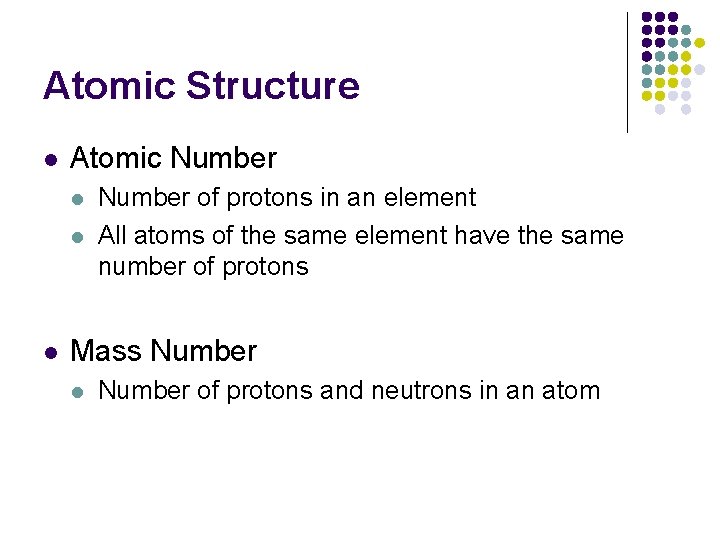
Atomic Structure l Atomic Number l l l Number of protons in an element All atoms of the same element have the same number of protons Mass Number l Number of protons and neutrons in an atom

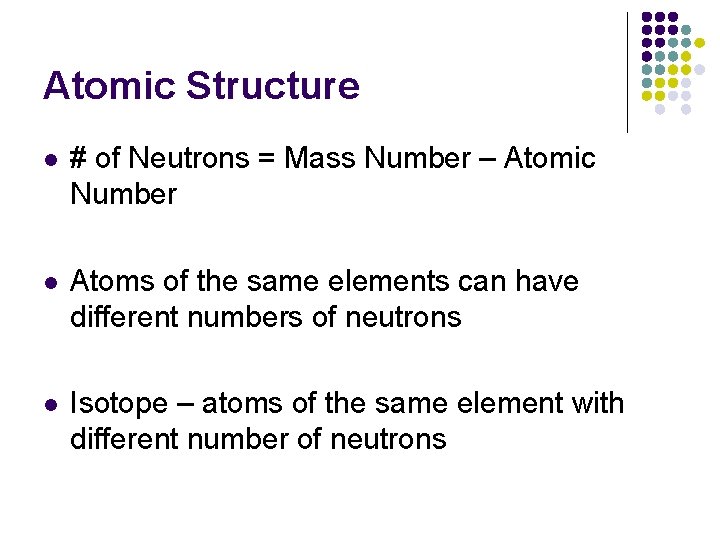
Atomic Structure l # of Neutrons = Mass Number – Atomic Number l Atoms of the same elements can have different numbers of neutrons l Isotope – atoms of the same element with different number of neutrons

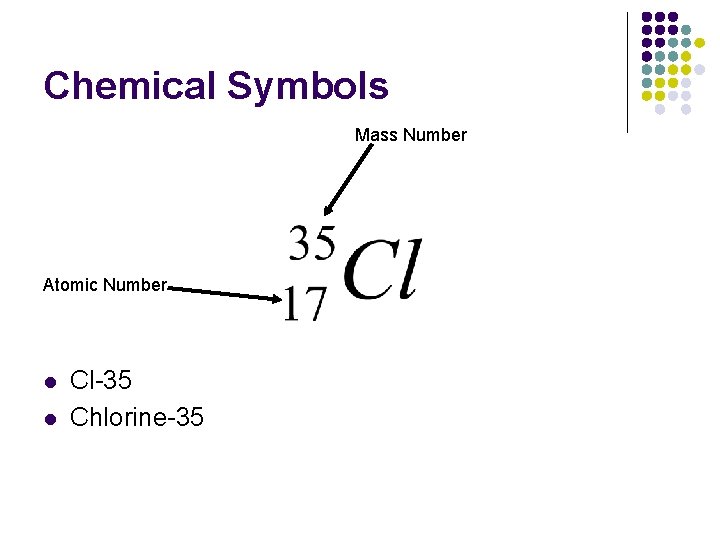
Chemical Symbols Mass Number Atomic Number l l Cl-35 Chlorine-35

Ion l Atom or group of atoms that have gained or lost one or more electrons l l Have a charge Example: l H+, Ca 2+, Cl-, OH-

Ions l H+ 1 proton 0 electrons l Ca 2+ 20 protons 18 electrons - 17 protons 18 electrons 9 protons 10 electrons l Cl l OH-


Atomic Theories l Rutherford’s model could not explain the chemical properties of elements l Niels Bohr believed Rutherford’s model needed to be improved l Bohr proposed that electrons are found only in circular paths around the nucleus

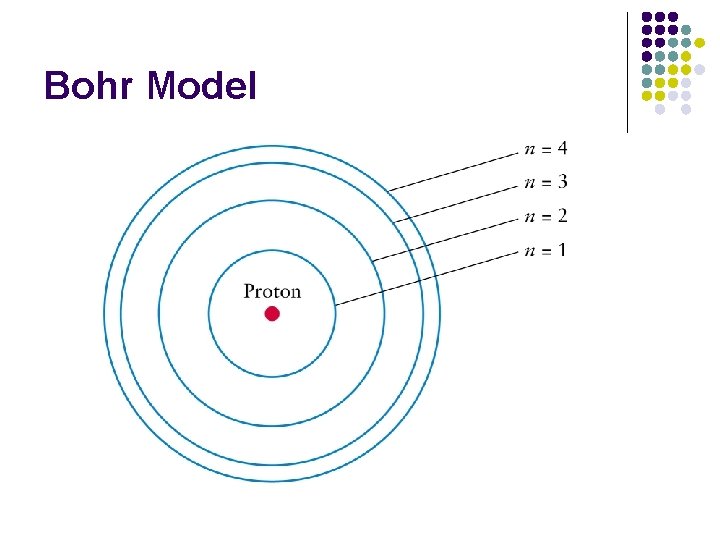
Bohr Model l Dense positive nucleus l Electrons in specified circular paths, called energy levels l These energy levels gave results in agreement with experiments for the hydrogen atom.

Bohr Model

Bohr Model l Each energy level can only hold up to a certain number of electrons l Level 1 2 electrons Level 2 8 electrons Level 3 18 electrons Level 4 32 electrons l l l

Electron Configuration l The way in which electrons are arranged in the atom Example: Na 2 -8 -1 l Valence Electrons l l Electrons in the outermost energy level

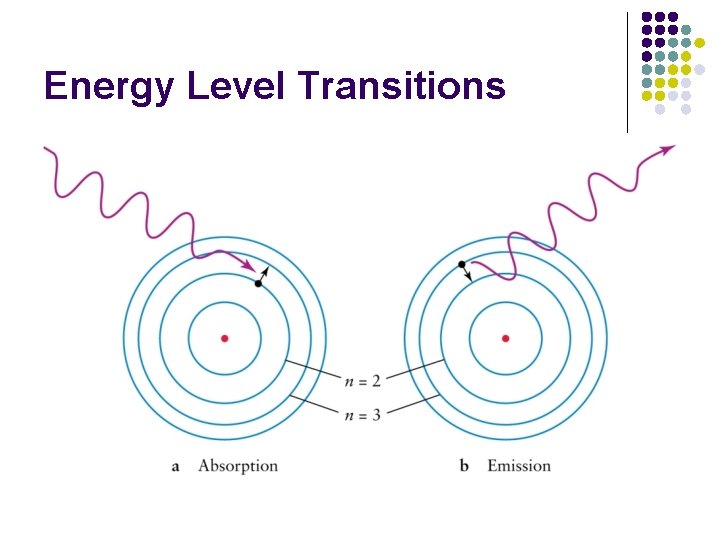
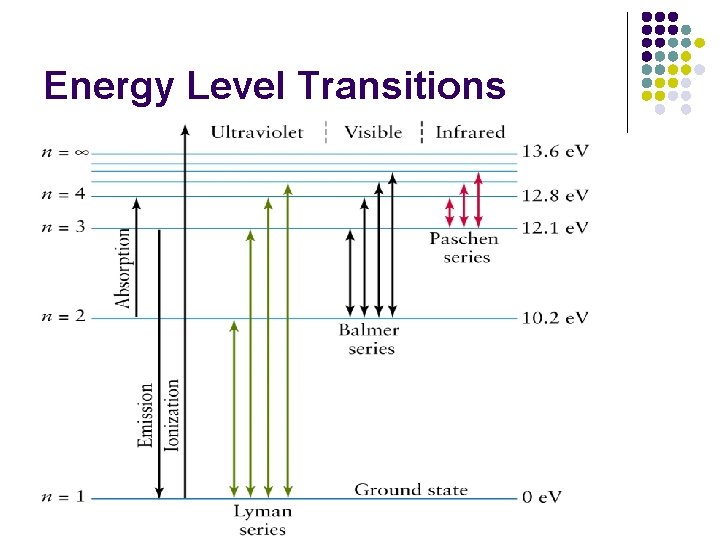
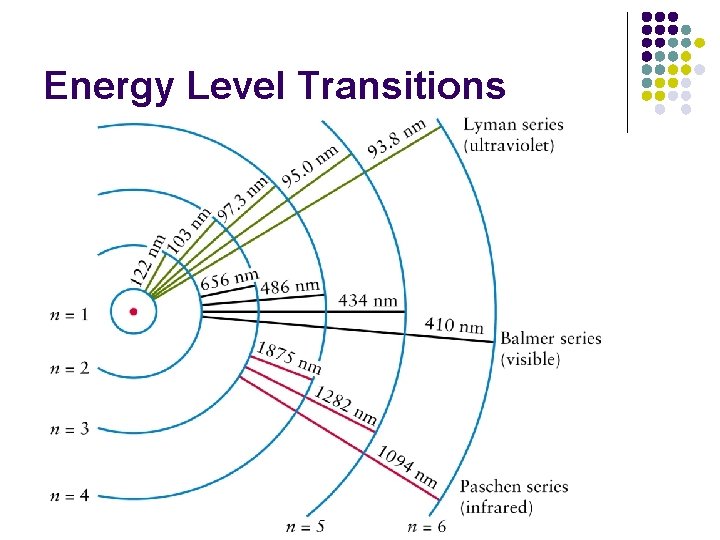
Energy Level Transitions l Electron energy is quantized l Electrons can move between energy levels with gains or losses of specific amounts of energy.

Energy Level Transitions l Gaining energy will move an electron outward to a higher energy level (Absorption) l When an electron falls inward to a lower energy level, it releases a certain amount of energy as light (Emission)

Energy Level Transitions

Ground State vs. Excited State l Ground State l l l When the electrons are in the lowest available energy level Ex: Na 2 -8 -1 Excited State l l When one or more electrons are not in the lowest available energy level Ex: Na 2 -7 -2 or 2 -8 -0 -1 or 2 -6 -1 -1 -1

Line Spectra l Emission Spectra l l Shows only the light that is emitted from an electron transition Absorption Spectra l Shows a continuous color with certain wavelengths of light missing (absorbed)

Energy Level Transitions

Energy Level Transitions


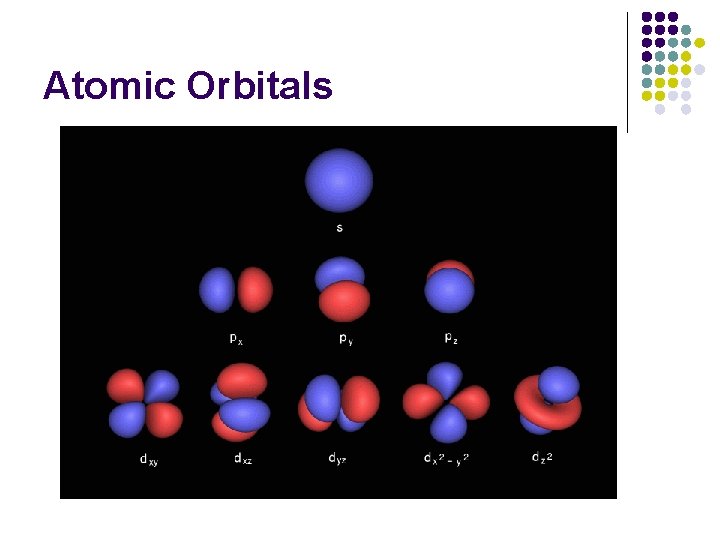
Wave Mechanical Model l More detailed view of the Bohr Model l Schrödinger Wave Equation and Heisenberg Uncertainty provides region of high probability where electron COULD be. l l Orbital Modern Model l AKA Quantum Mechanical Model, Electron Cloud Model

Wave Mechanical Model l Orbital l Regions of space where there is a high probability of finding an electron

Wave Mechanical Model l Each energy level is divided into sublevels l l l 1 st Energy level has 1 sublevel, s 2 nd Energy level has 2 sublevels, s and p 3 rd Energy level has 3 sublevels, s, p, and d 4 th Energy level has 4 sublevels, s, p, d, and f These sublevels start to overlap as you move away from the nucleus

Wave Mechanical Model l Sublevels are divided into orbitals l l l s sublevel has 1 orbital p sublevel has 3 orbitals d sublevel has 5 orbitals f sublevel has 7 orbitals Each orbital can hold up to 2 electrons

Atomic Orbitals

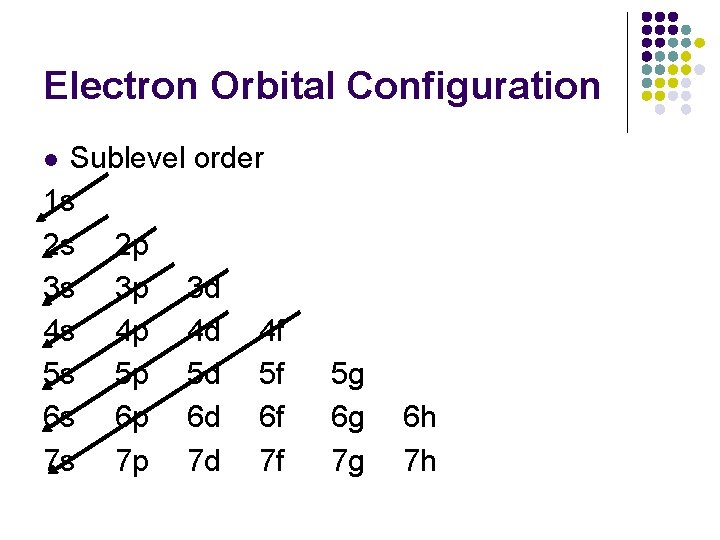
Electron Orbital Configuration Sublevel order 1 s 2 s 2 p 3 s 3 p 3 d 4 s 4 p 4 d 4 f 5 s 5 p 5 d 5 f 6 s 6 p 6 d 6 f 7 s 7 p 7 d 7 f l 5 g 6 g 7 g 6 h 7 h

Electron Orbital Configuration l One sublevel must be full before you can move to the next sublevel l For sublevels with multiple orbitals l Each orbital must have one electron before you can double up

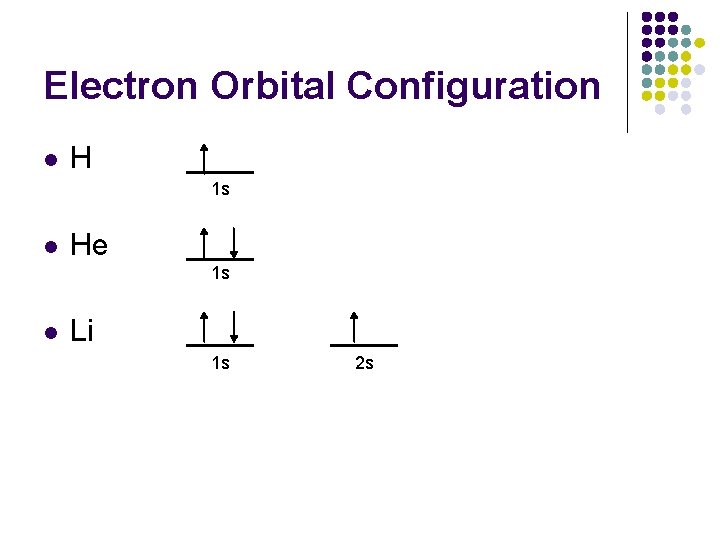
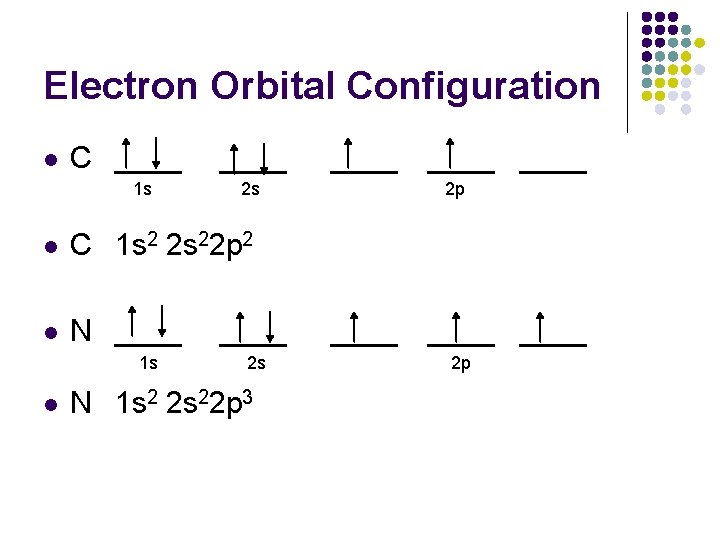
Electron Orbital Configuration l H ____ 1 s 1 1 s l He ____ 1 s 2 1 s l Li ____ 1 s 2 s 1 s 2 2 s 1

Electron Orbital Configuration l C ____ 1 s ____ 2 s l C 1 s 2 2 s 22 p 2 l N ____ 1 s l ____ 2 s N 1 s 2 2 s 22 p 3 ____ 2 p ____ 2 p

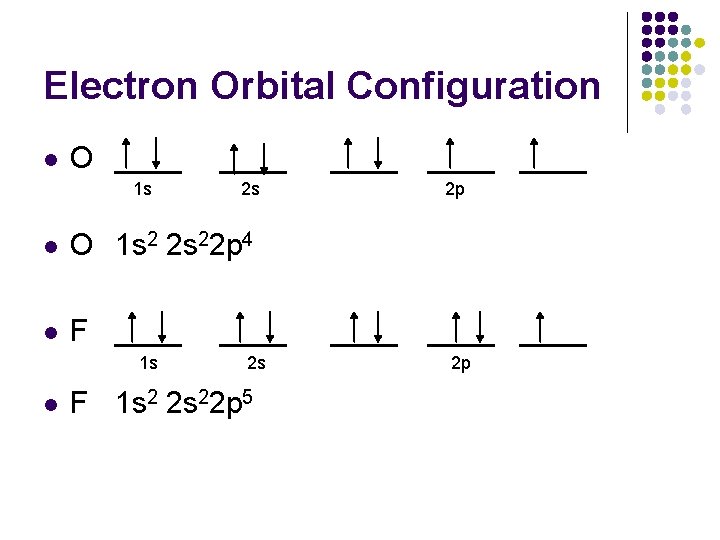
Electron Orbital Configuration l O ____ 1 s ____ 2 s l O 1 s 2 2 s 22 p 4 l F ____ 1 s l ____ 2 s F 1 s 2 2 s 22 p 5 ____ 2 p ____ 2 p


M&M’s Demo l What colors are found in a regular M&M’s bag? l l l Green Yellow Orange Blue Red Brown

M&M’s Demo l Do you get an equal amount of each color in each bag? l If we opened up all the regular M&M bags in the world would we get an equal number of each color? l Are you supposed to?

M&M’s Demo Color 1 bag World Blue % 24% Green % 16% Yellow % 14% Orange % 20% Red % 13% Brown % 13%

M&M’s Demo l M&M’s come in certain abundances (percentages) So do isotopes of each element l Relative Abundance l l Percent of each naturally occurring isotope found in nature

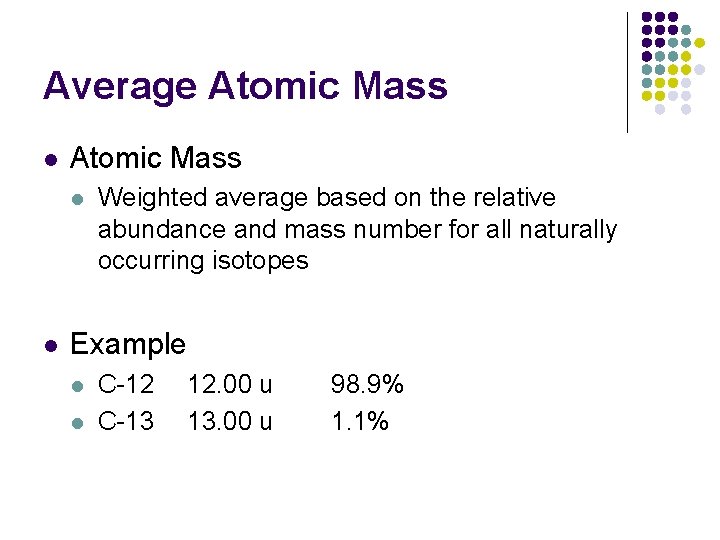
Average Atomic Mass l l Weighted average based on the relative abundance and mass number for all naturally occurring isotopes Example l l C-12 C-13 12. 00 u 13. 00 u 98. 9% 1. 1% 12. 011 u

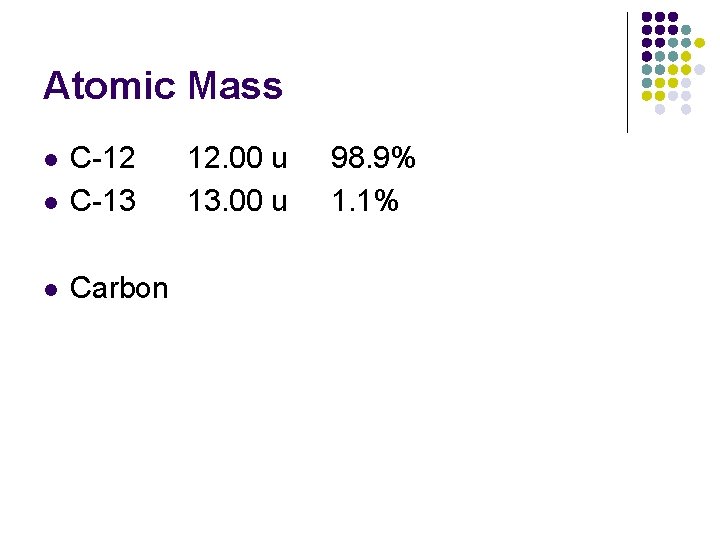
Atomic Mass l l C-12 C-13 12. 00 u 13. 00 u 98. 9% 1. 1% Carbon 0. 989 x 12. 00 + 0. 011 x 13. 00 = 12. 011 u l