Atomic Theory a scientific theory of the nature










- Slides: 32

Atomic Theory -a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms.

Democritus -influential pre. Socratic philosopher -formulated an atomic theory -400 B. C.

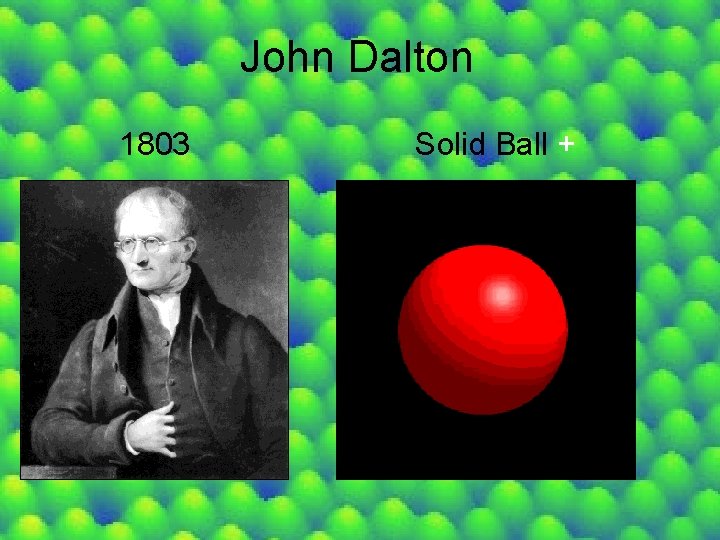
John Dalton 1803 Solid Ball +

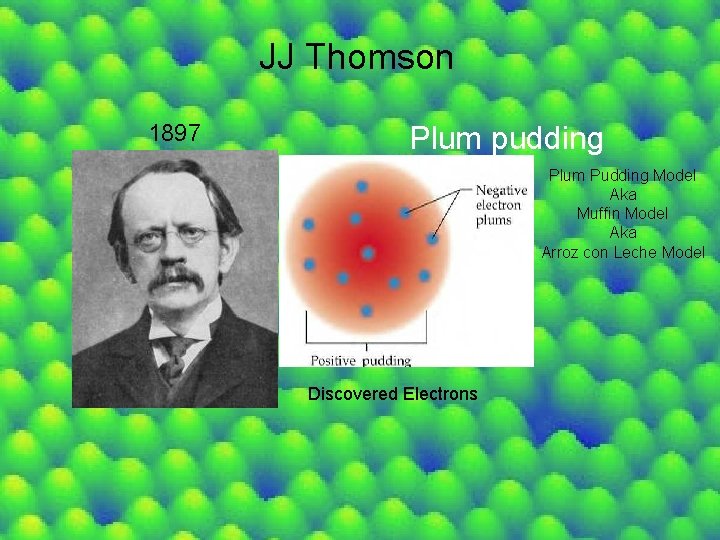
JJ Thomson 1897 Plum pudding Plum Pudding Model Aka Muffin Model Aka Arroz con Leche Model Discovered Electrons

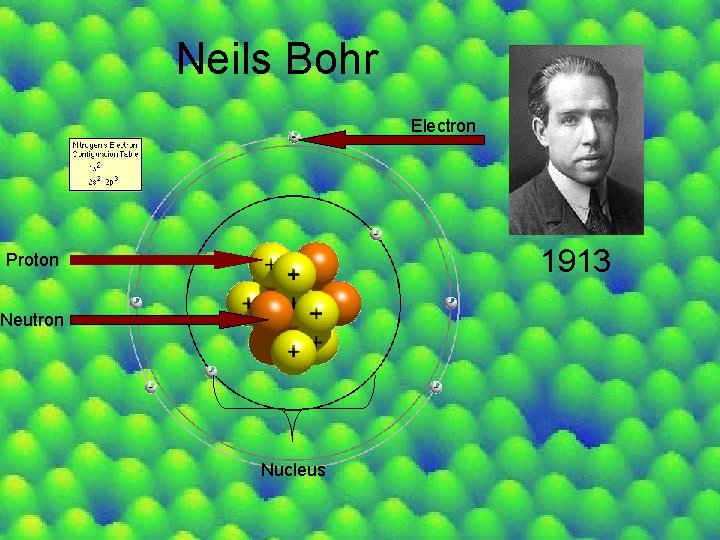
Neils Bohr Electron 1913 Proton Neutron Nucleus

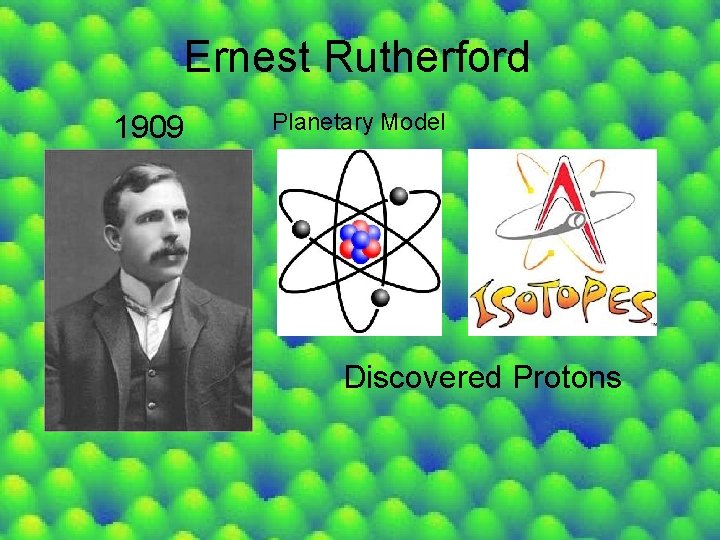
Ernest Rutherford 1909 Planetary Model Discovered Protons

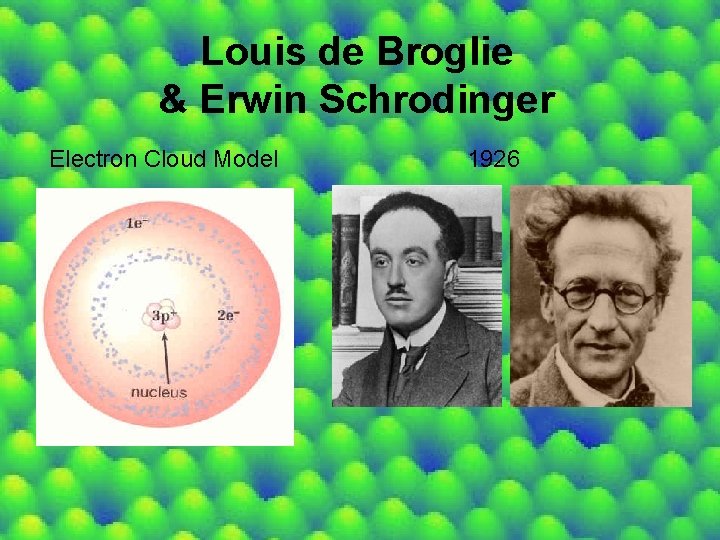
Louis de Broglie & Erwin Schrodinger Electron Cloud Model 1926

1927 Solvay Conference

Atomic Structure Notes

Element -substances that are the building blocks of all matter -made up of one kind of atom -Ex. Carbon, Hydrogen, Oxygen

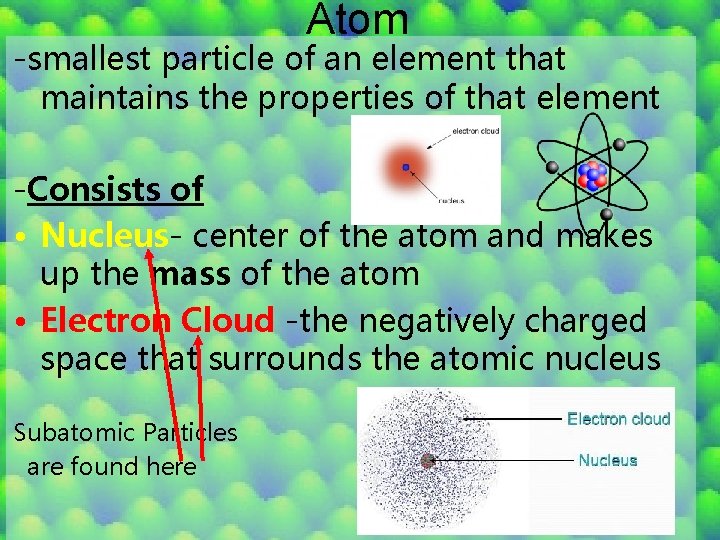
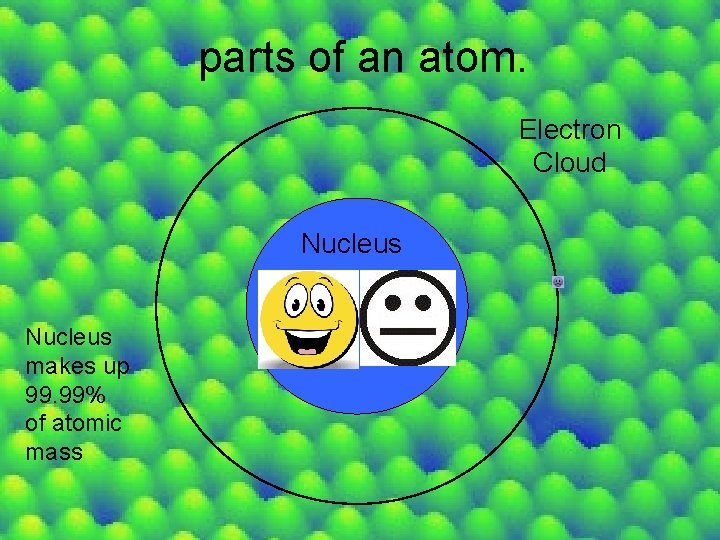
Atom -smallest particle of an element that maintains the properties of that element -Consists of • Nucleus- center of the atom and makes up the mass of the atom • Electron Cloud -the negatively charged space that surrounds the atomic nucleus Subatomic Particles are found here

Subatomic Particles -a particle smaller than an atom 3 main Subatomic Particles 1. Protons 2. Neutrons 3. Electrons

Protons (+) • particles found in the NUCLEUS of an atom. • have a POSITIVE charge

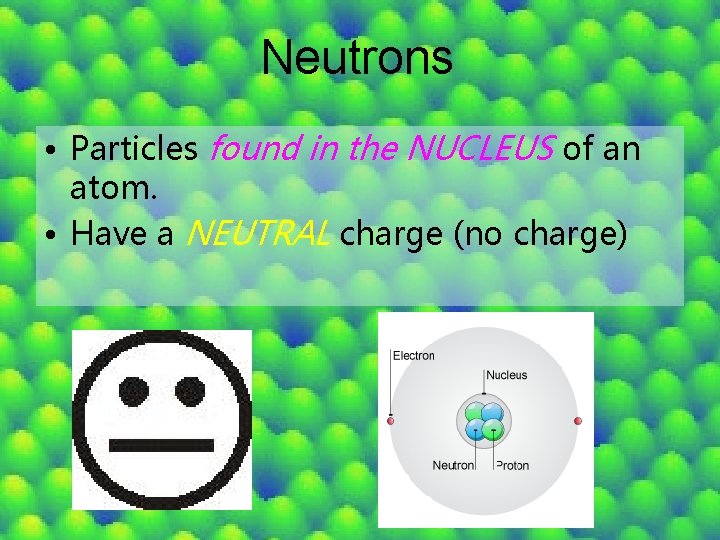
Neutrons • Particles found in the NUCLEUS of an atom. • Have a NEUTRAL charge (no charge)

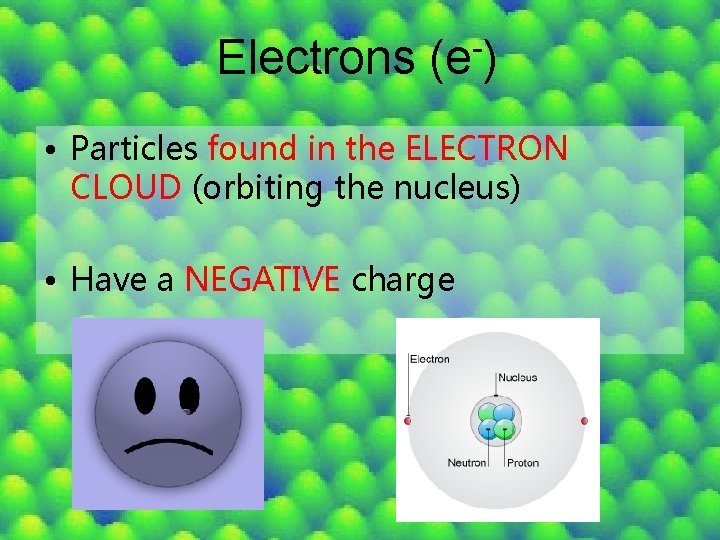
Electrons (e ) • Particles found in the ELECTRON CLOUD (orbiting the nucleus) • Have a NEGATIVE charge

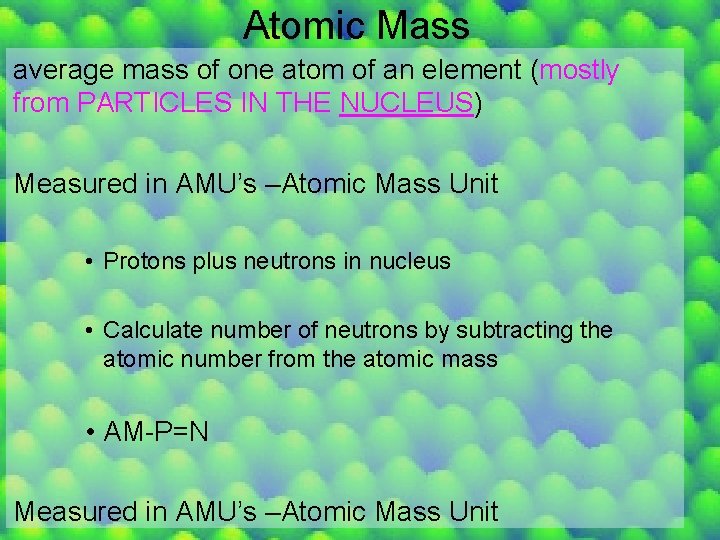
Atomic Mass average mass of one atom of an element (mostly from PARTICLES IN THE NUCLEUS) Measured in AMU’s –Atomic Mass Unit • Protons plus neutrons in nucleus • Calculate number of neutrons by subtracting the atomic number from the atomic mass • AM-P=N Measured in AMU’s –Atomic Mass Unit

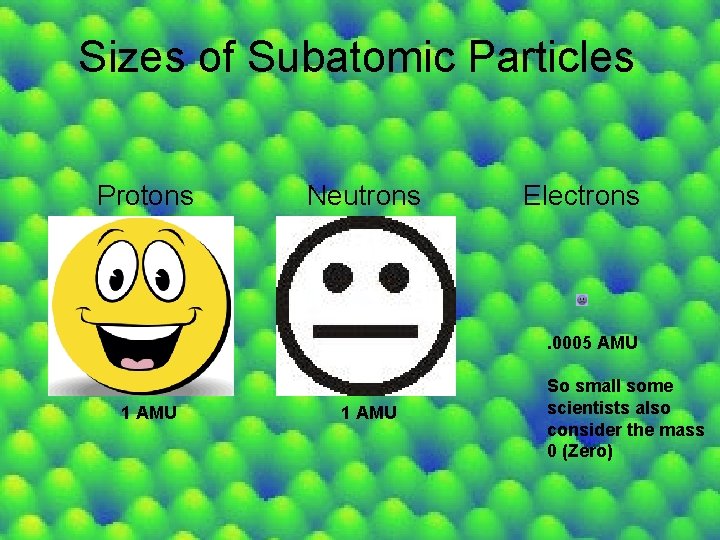
Sizes of Subatomic Particles Protons Neutrons Electrons . 0005 AMU 1 AMU So small some scientists also consider the mass 0 (Zero)

parts of an atom. Electron Cloud Nucleus makes up 99. 99% of atomic mass

Atomic Number -reflects the # of protons in the nucleus and electrons in the electron cloud of a balanced atom of that element. ATOMIC NUMBER = # OF PROTONS & ELECTRONS

Atomic Number

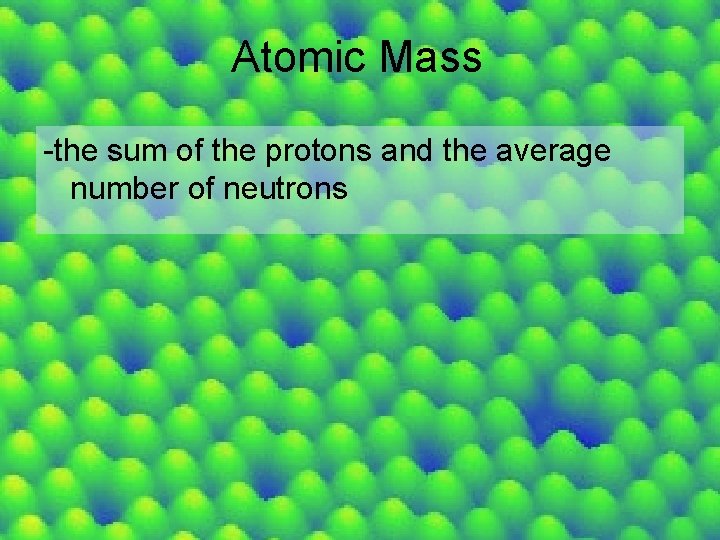
Atomic Mass -the sum of the protons and the average number of neutrons

Atomic Mass

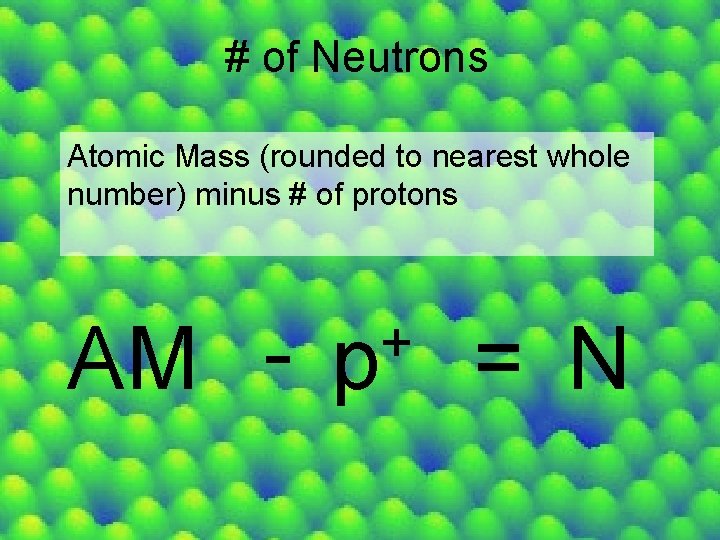
# of Neutrons Atomic Mass (rounded to nearest whole number) minus # of protons AM - + p = N

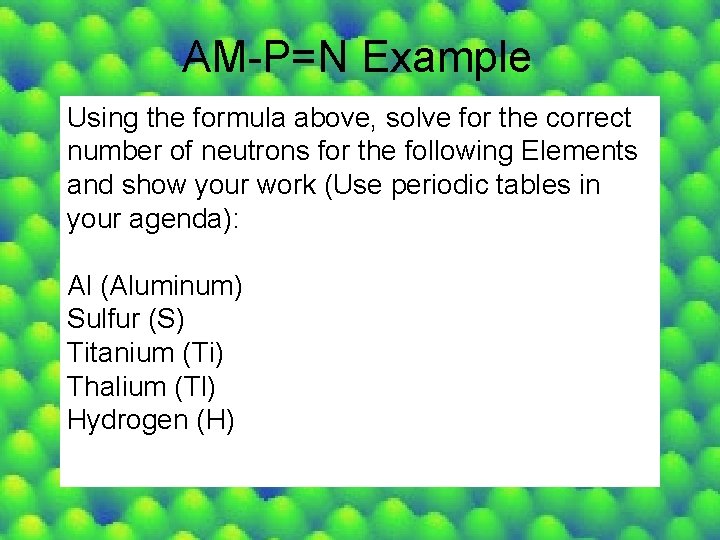
AM-P=N Example Using the formula above, solve for the correct number of neutrons for the following Elements and show your work (Use periodic tables in your agenda): Al (Aluminum) Sulfur (S) Titanium (Ti) Thalium (Tl) Hydrogen (H)

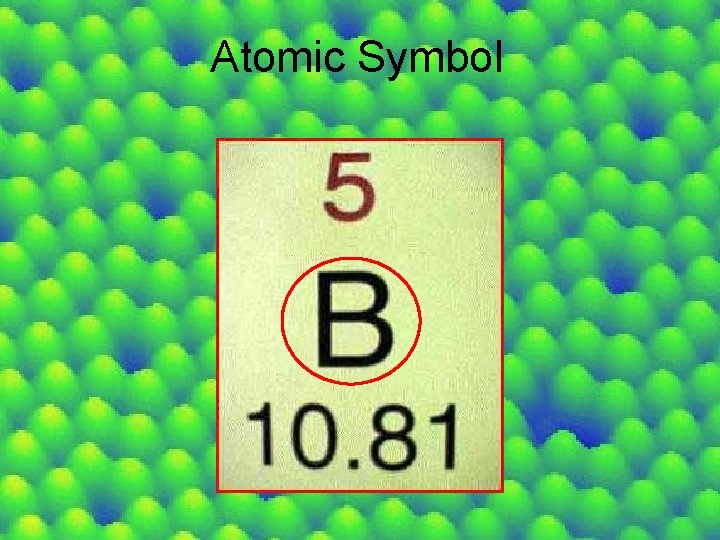
Atomic Symbol

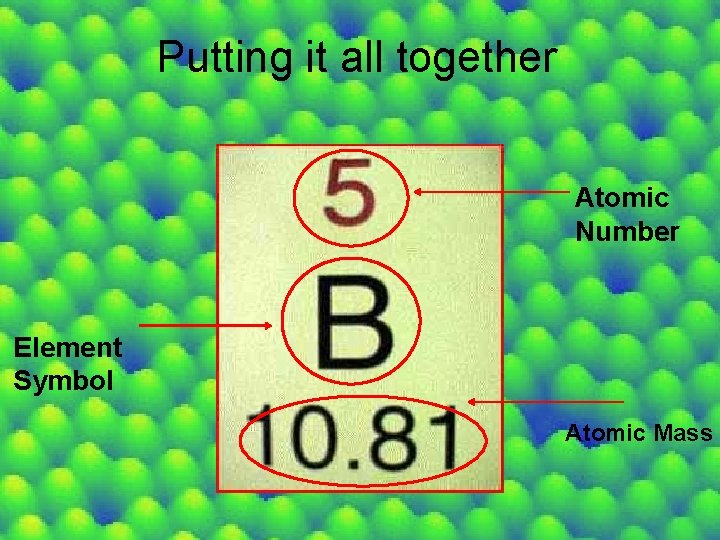
Putting it all together Atomic Number Element Symbol Atomic Mass

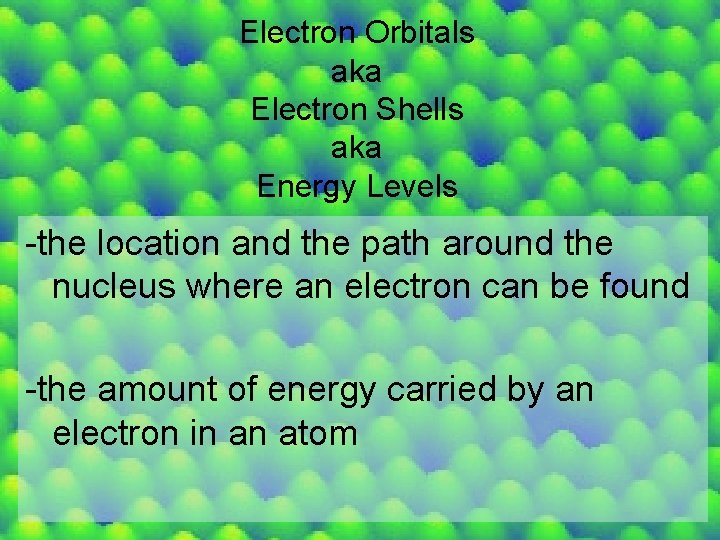
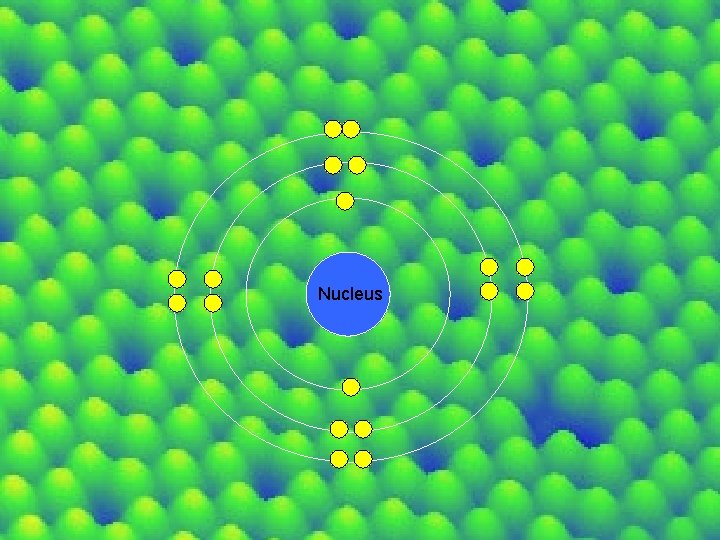
Electron Orbitals aka Electron Shells aka Energy Levels -the location and the path around the nucleus where an electron can be found -the amount of energy carried by an electron in an atom

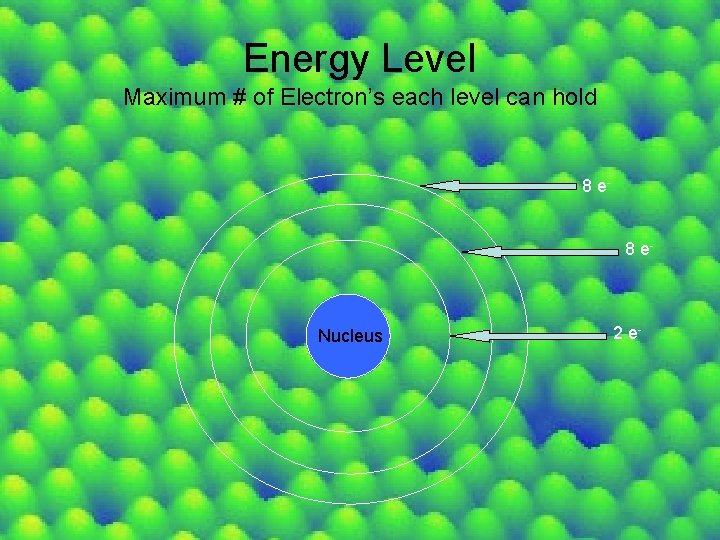
Energy Level Maximum # of Electron’s each level can hold 8 e- Nucleus 2 e-

Nucleus

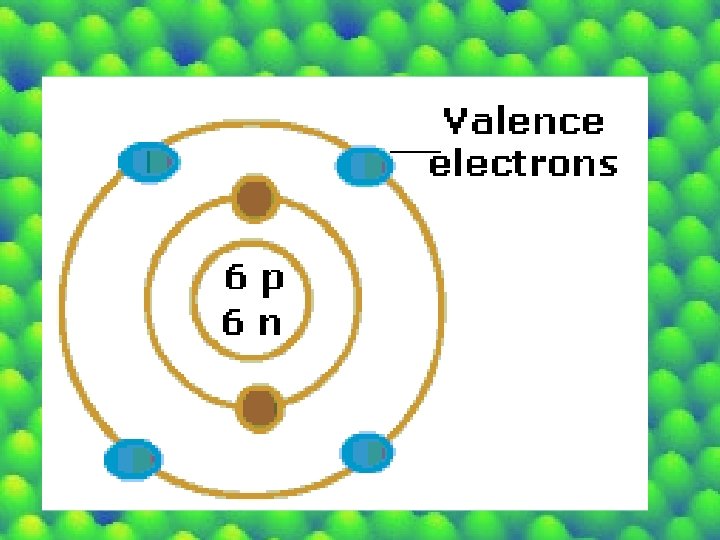
Valence Shell -the outermost shell of an atom in its uncombined state -all atoms want their valence shells filled.

Valence Electron • electrons located in the valence shell • # of valence e- will determine the reactivity of the atom. • The more Valence e’s the more stable and HAPPY (Noble Gases) • The less valence e’s the more reactive and EXPLOSIVE or UNHAPPY (Alkali Earth Metals)
