Atomic Theory 1132018 Scientistmel com Twitter comscientistmel Patreon





















- Slides: 28

Atomic Theory 11/3/2018 Scientistmel. com Twitter. com/scientistmel Patreon. com/scientistmel

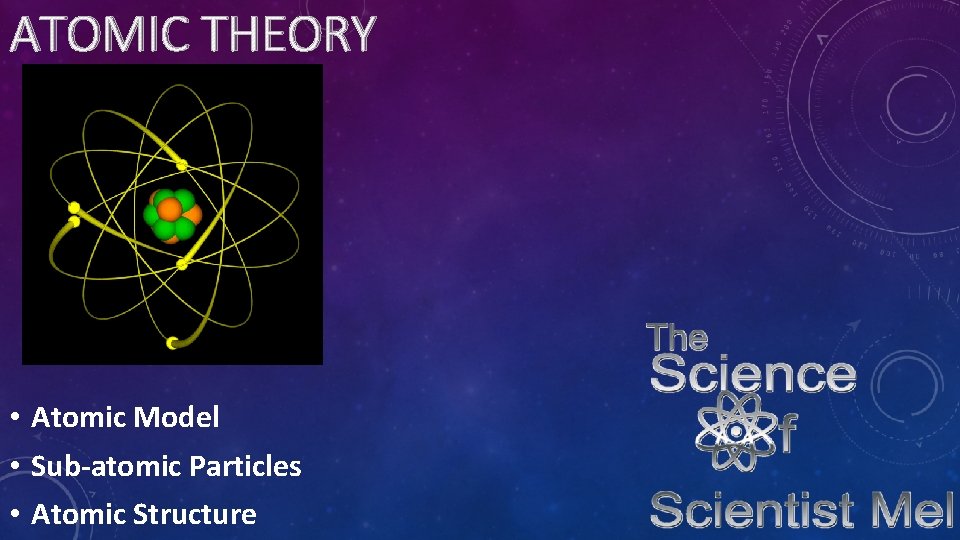
ATOMIC THEORY • Atomic Model • Sub-atomic Particles • Atomic Structure

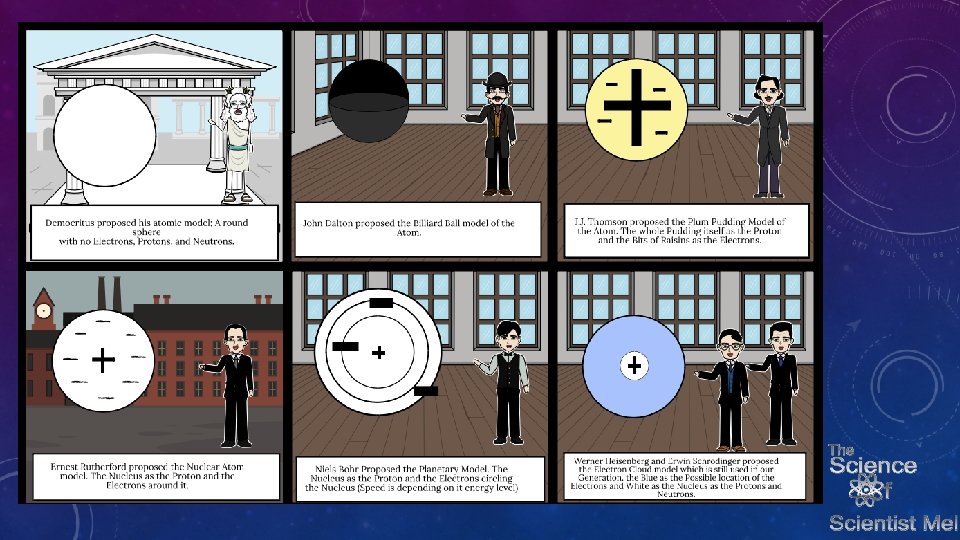
ATOMS • Atomic models have changed over time. The model will likely continue to change when we get new information. In order to understand how the model has changed, we must go back to Democritus and early Greek philosophers.

ATOMS

ATOMS • Democritus believed that atoms made up everything but were mostly empty space. He believed atoms held essentially the same characteristics as what they made up • Iron made of iron atoms, cheese made of cheese atoms,

ATOMS

ATOMS • In 1803, John Dalton proposed the term atom as he proposed that atoms were indivisible. He drew from Democritus in the idea of the atom being like a billiard ball. Where he differed with Democritus was that elements differ from other elements. Dalton however was wrong about atoms not being indivisible. They are as they are made of particles.

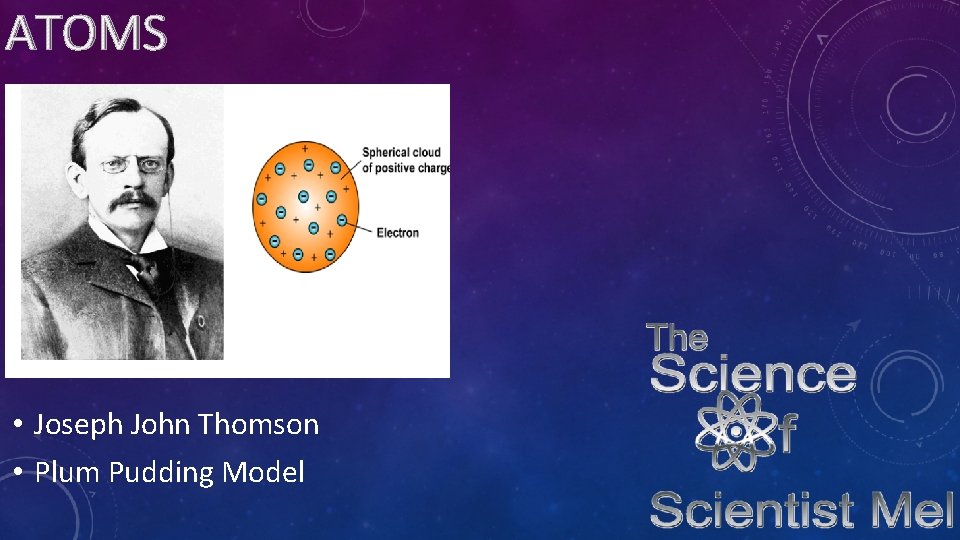
ATOMS • Joseph John Thomson • Plum Pudding Model

ATOMS • In 1904, Thomson using cathode rays discovered the existence of electrons. He first called them corpuscles. He won the nobel prize for his discovery. His model shows the electrons scattered out in a could…which is correct…but what he did not know is that there is a positively charged nucleus in an atom. His model is called the plum pudding model because it resembles the dish “plum pudding” with bits of fruit spread out in it.

ATOMS • Ernest Rutherford • “Now I know what the atom looks like”

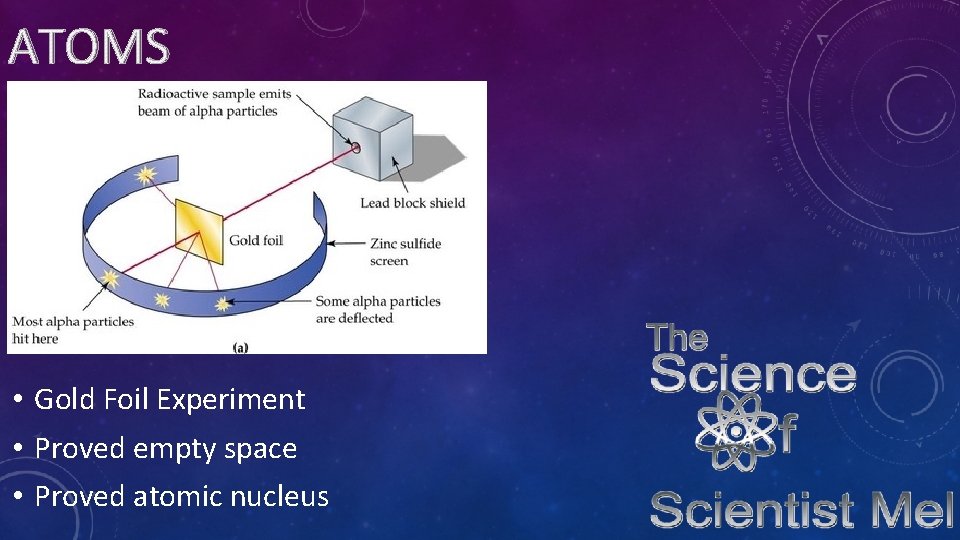
ATOMS • Gold Foil Experiment • Proved empty space • Proved atomic nucleus

ATOMS • Rutherford Model

ATOMS • Rutherford got it right about the nucleus. . protins and neutrons…he also got it right about electrons orbiting the nucleus…but what he didn’t explain is how it is that electrons remain in orbits around the nucleus…enter a the epic, exciting, and BOHR-ing Neils Bohr and his planetary model…see what I did there!

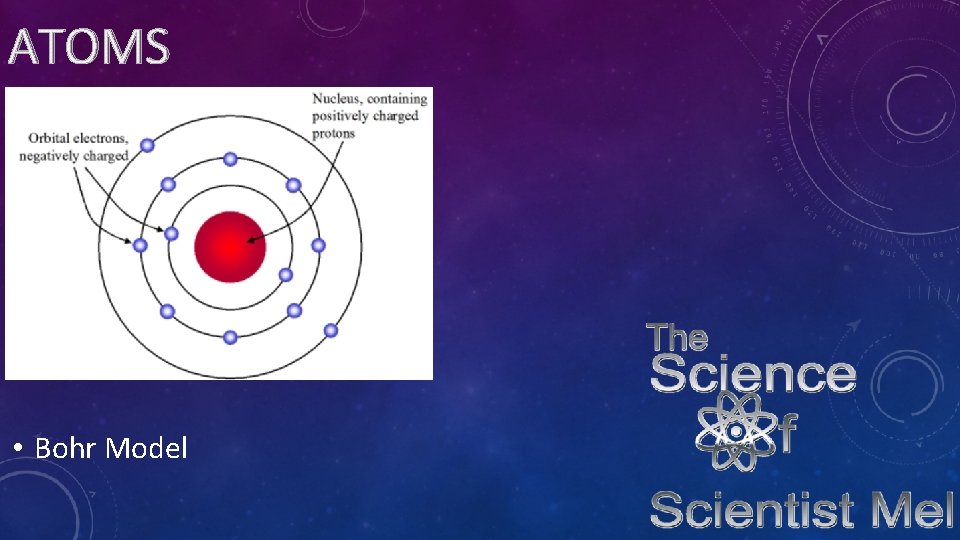
ATOMS • Niels Bohr • Put electrons in orbit • Explained spectral lines

ATOMS • Bohr Model

ATOMS

ATOMS • Bohr’s work gave a better understanding as to how light affects atoms. Electrons get excited when hhit with certain wavelengths of light. This led to research into how different elements have their own fingerprint signatures in what wavelengths of light they release when their electrons relax. What Bohr got right were the electrons in energy levels…but it doesn’t really explain how heavier atoms don’t have their electrons completely collapse into their massive nuclei full of protons and neutrons…Now we see Schrodinger jumping into the equation…

ATOMS • Quantum Model

ATOMS • Schrodinger along with Werner Heisenberg in 1926 proposed that electrons (like light) move in waves. Heisenberg and his uncertainty principal discusses that the more you know about the location of a particle, the less you know about the momentum (and vice versa). Schrodinger used thought experiments to also discuss that just by observing the universe, you change its behavior. Light affects electrons…so we can not possibly know everything. So how to solve this atomic model conundrum? • They used math in order to determine the probability that an electron is in a particular area…this gave a 3 -dimensional structure to the different orbitals of an atom. His equaitons take into account 3 coordinates, pribcipal, angular, and magnetic quantum numbers. These numbers describe the shape, size, and orientation in space of the orbitals of an atom. It is our best model to date for describing the atom.

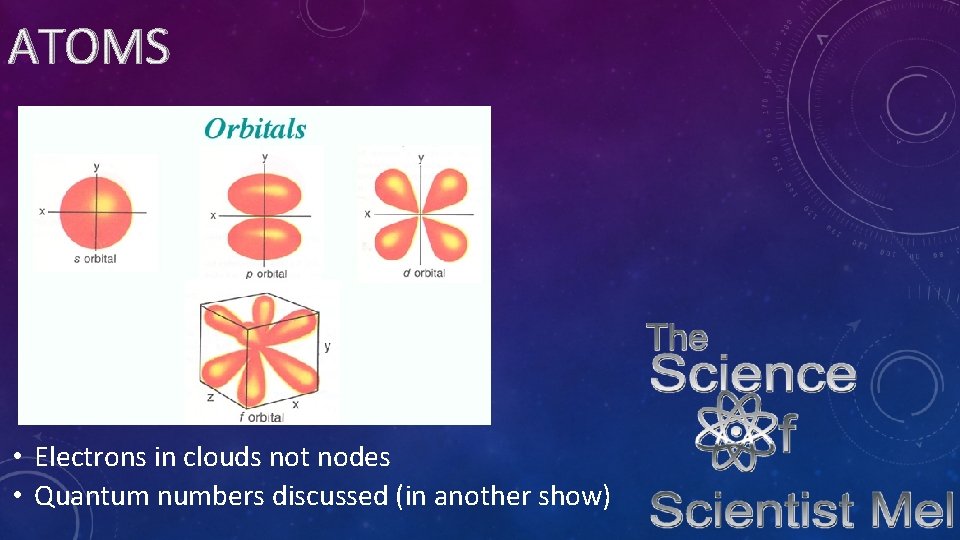
ATOMS • Quantum Model

ATOMS • Electrons in clouds not nodes • Quantum numbers discussed (in another show)


ATOMS • The model of the atom has changed throughout the years…and it can change again when we get more information…and that is ok. So far, the current model is working well with what we know in our natural world. • If you want to learn more about atomic theory, hit me up on twitter, in the comments here, or my facebook page. I haven’t listed any sources for this particular episode as there is so much information on the internet and all of this is textbook material!

ATOMIC THEORY • Atomic Model • Sub-atomic Particles • Atomic Structure

THANK YOU TO MY PATRONS • • • Paola • Tim • Andy • Keri • Tony • Godless Iowan Carl • Circe • Bo • Jennifer Melanie • Keith • Steven • Corey • Duke • James • Sarah • Richard Toni • James Lauren Jenn Patrick Daniel Steven • • Graham Zachary • Dragnaucht Chris

You can find me… • Scientist. Mel. com • Patreon. com/scientistmel • Pscp. tv. com/scientistmel • Youtube. com/scientistmel • Facebook. com/scientistmel

Atomic Theory 11/3/2018 Scientistmel. com Twitter. com/scientistmel Patreon. com/scientistmel

• https: //www. livescience. com/52592 -spooky-effects-sleep-deprivation. html • https: //www. theparanormalmd. com/types-of-hauntings-entities. html • https: //www. mayoclinic. org/diseases-conditions/temporal-lobe-seizure/expert-answers/phantosmia/faq 20058131 • https: //chipmusic. org/forums/topic/11665/digital-recording-equipment-picking-up-radio-stations/ • https: //www. sciencedaily. com/releases/2015/03/150331121251. htm • https: //www. ncbi. nlm. nih. gov/pmc/articles/PMC 4560180/#bib 7 • https: //www. nhs. uk/conditions/sleep-paralysis/ • https: //www. livescience. com/25448 -pareidolia. html • https: //www. scientificamerican. com/article/patternicity-finding-meaningful-patterns/ • http: //www. who. int/peh-emf/about/Whatis. EMF/en/index 3. html