Atomic Theory 1 What is the Atomic theory










- Slides: 11

Atomic Theory 1. What is the Atomic theory? 2. What are the main sections of an atom? 3. What are the different subatomic particles? 4. How do the different particles interact?

What is the atomic theory? All matter in the universe is made of atoms Atom: The smallest unit of matter that retains(continue to have) the identity of the substance. If we break an atom up into smaller parts, the identity of the substance is lost. First proposed by a guy named Democratus

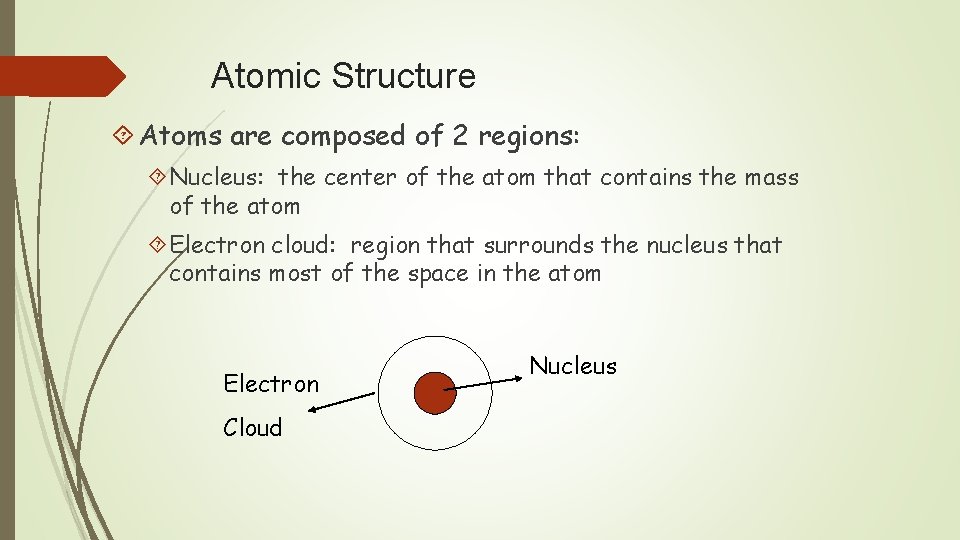
Atomic Structure Atoms are composed of 2 regions: Nucleus: the center of the atom that contains the mass of the atom Electron cloud: region that surrounds the nucleus that contains most of the space in the atom Electron Cloud Nucleus

Part 1: The Nucleus: Contains 2 subatomic particles Subatomic: things that are smaller than the atom Protons (p+): Positivity charged subatomic particle Protons are like the friends you want in your life. Neutrons (no): neutrally charged (No charge) subatomic particles These are the friends that just exist in your life.

What is in the Electron Cloud? ? Electrons!!!! The 3 rd particle resides in a could of sorts that is located around the nucleus. Electron (e-): the subatomic particle with a negative charge. The friend you don’t want around. Electrons have nearly no mass

The Nucleus Protons and Neutrons are both located in the nucleus. The over all charge of the nucleus is positive. This is also where nearly all of the mass is located. The Electron Cloud Is negatively charged Has next to no mass but nearly all the volume of an atom

What is the overall charge of a normal atom? Nucleus is positive Electron cloud is negative Neutrons have no charge (they do not have to equal the number of the other subatomic particles. In a normal atom: Protons = electrons If there are 10 protons there are 10 electrons

Atomic Number and Atomic Mass Each Element has a specific Atomic Number and Atomic Mass Atomic Number is the number that indicates the number of protons in an atom Ex. Hydrogen’s atomic number is 1 so there is 1 proton in the atom Ex. 2 Nitrogen atomic number is 7 so there are 7 protons in the atom ***VERY IMPORTANT: : The number of protons identifies the atom.

Atomic Mass and the number of Neutrons Atomic Mass number: the number of protons and neutrons in the nucleus Example Hydrogen can have a mass of 3. Since it has 1 proton it must have 2 neutrons # of neutrons = mass # - atomic #

Finding the number of Protons and Neutrons Lithium (Li) has a mass number of 7 and an Atomic Number of 3 Protons = 3 (same as atomic number) Neutrons = 7 – 3 = 4 (atomic mass # - atomic #) Neon (Ne) has a mass number of 20 and an atomic number 10 Protons = 10 Neutrons 20 -10 = 10

Electrons The electrons are equal to the number of protons So electrons = protons = atomic number Ex Fluorine (F) has an atomic number of 9 and atomic mass of 19 P+ =9 N 0 = 10 E - = 9