ATOMIC STRUCTURES OF MINERALS AND IONIC SUBSTITUTION ATOMIC

ATOMIC STRUCTURES OF MINERALS AND IONIC SUBSTITUTION
ATOMIC STRUCTURES OF MINERALS • Stable atomic structures in minerals can be explained largely by Pauling’s Rules ( 1 -4) • These rules assume atoms act as spheres and there is a strong ionic bond between atoms • Pauling’s Rules • 1. (Rule 1)--Coordination Number (CN) Principle • a specific number of anions surround each cation (CN) resulting in a geometric configuration • radius ratio (rr) = (ionic radius of cation / ionic radius of anion) and determines the CN • CN determines the geometric configuration of anions around the cation
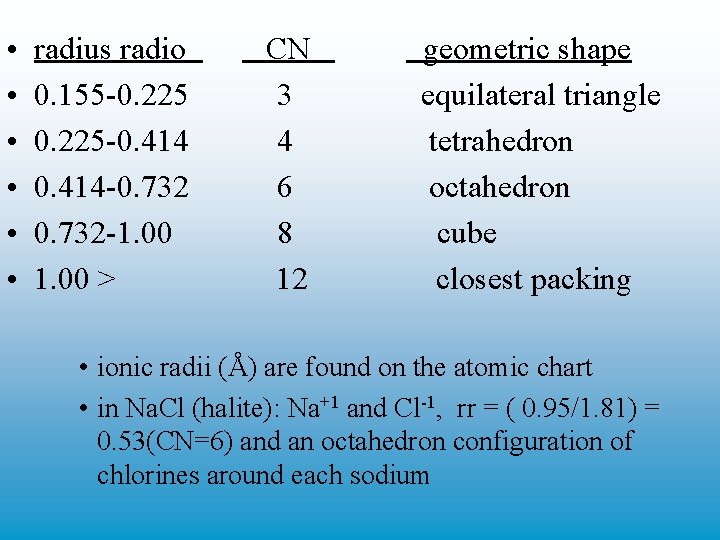
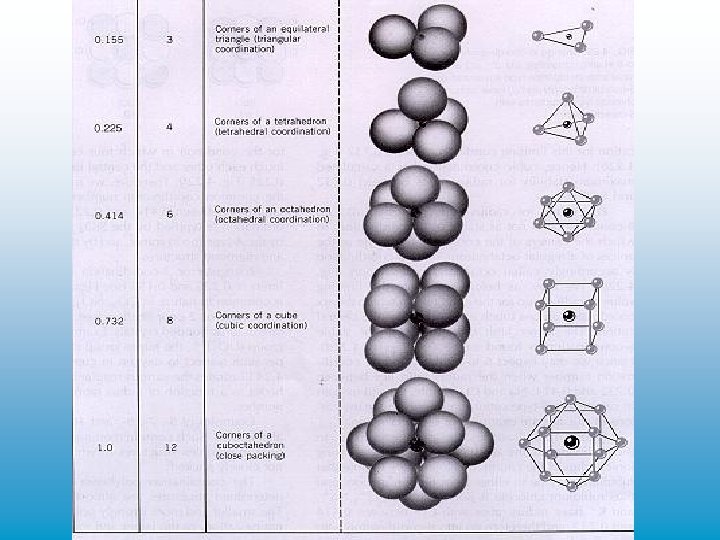
• • • radius radio 0. 155 -0. 225 -0. 414 -0. 732 -1. 00 > CN 3 4 6 8 12 geometric shape equilateral triangle tetrahedron octahedron cube closest packing • ionic radii (Å) are found on the atomic chart • in Na. Cl (halite): Na+1 and Cl-1, rr = ( 0. 95/1. 81) = 0. 53(CN=6) and an octahedron configuration of chlorines around each sodium
• the calculated and actual CN may differ based on 2 conditions • 1. if rr is borderline between CN, CN the temperature or pressure of mineral formation cause this difference • a high temperature of formation (as in a magma), could cause the lower CN for the bond and the higher CN if mineral formed at a low temperature • the pressure of formation is directly proportional to the same result above--- but pressure is not as important as temperature • 2. if % ionic character of the bond is too low-low this would favor non spherical geometry--use of the molecular orbital theory or molecular hybridization concepts may result in the true CN for a bond if it is believed # 2, may give unreliable results
• in the example above for Na. Cl, there should be a high confidence level for the calculated 6 CN for the Na--Cl since • the rr of 0. 53 is not borderline • the % ionic character for the bond is high (67%)---see periodic table for determination of % ionic character of a bond from electronegativities • 2. (Rule 2)--Electrostatic valence principle • e. v. number of a single bond = (absolute valence number of cation(z) / CN ) • the total electrostatic valence numbers reaching a centrally coordinated cation or anion equal the valence number of that central ion
• in a mineral in which Ca+2 is centrally coordinated, the sum of all individual ev values attached to Ca must equal 2 • likewise, in a mineral in which F-1 is centrally coordinated, all individual ev values must equal 1 • note that applying rule 2, will (1) confirm the number of anions around the cation obtained from rule 1, and (2) determine the number of cations surrounding the anion • Applying rules 1 and 2, determine the structure of hematite, Fe 2 O 3 • for the Fe+3 -O-2 bond---from rule 1, rr = (0. 65/1. 40) = 0. 46= 6 CN---from rule 2: ev = 3/6 = 1/2 and there must be 6, 1/2 bonds coordinating Fe to equal the +3 valence; and there must be 4 Fe surrounding the O

• there are 3 categories of bond types related to the ev concept • isodesmic bond • a bond in which the ev value is less than 1/2 the valence number of the anion--the Na--Cl bond is an example • mesodesmic bond • a bond in which the ev value is equal to 1/2 the valence number of the anion-- the Si+4 --O-2 bond is an example: Si-O = 4 CN from rule 1 and ev =4/4 =1 from rule 2 • presence of this kind of bond allows minerals to possess polymerization structures • the following table from the textbook shows this


• aniosodesmic bond • a bond in which the ev value is more than 1/2 the valence number of the anion • an example is the C+4 --O-2 bond in carbonate minerals: C-O = 3 CN from rule 1: ev = 4/3 from rule 2 and this value is greater than 1/2 the absolute valence number of the anion (1) • the presence of this bond forms complex anions known as oxyacid anions or chemical radicals • the subscript number related to the O in this bond states the number of O coordinated to the cation-hence (1. ) a rr borderline problem and (2. ) the % ionic character of the bond do not have to be considered

• Table relating the name of mineral classes with anisodesmic anionic groups and CN • bond mineral class anionic group CN • S-O sulfate (SO 4) -2 4 • N-O nitrate (NO 3) -1 3 • C-O carbonate (CO 3) -2 3 • P-O phosphate (PO 4) -3 4 • V-O vanadate (VO 4) -3 4 • Mo-O molybdate (Mo. O 4) -2 4 • As-O arsenate (As. O 4) -3 4 • W-O tungstate (WO 4) -2 4

• the ev value reflects the relative strength of a bond-hence cleavage through mesodesmic and anisodesmic bonds is unlikely while that for isodesmic is more likely • 3. (Rule 3)--Sharing of polyhedra--1 • explains how polyhedra fit together in the atomic array of a mineral • the polyhedra fit is most stable if they are connected at points, since in this configuration there is the least repulsion between cations • sharing of polyhedra at edges and particularly faces results in greater cation repulsion caused by the closer position of cations
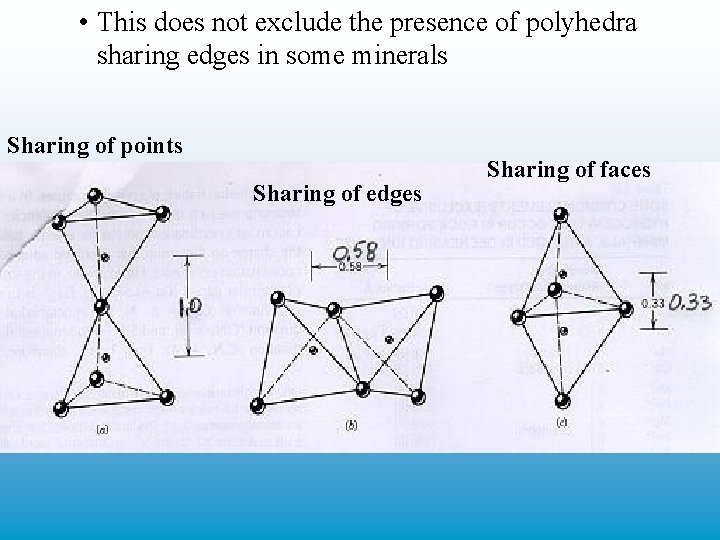
• This does not exclude the presence of polyhedra sharing edges in some minerals Sharing of points Sharing of edges Sharing of faces

• Relationship of unit cells to geometric units
• 4. (Rule 4)--Sharing of polyhedra--2 • in minerals containing different cations, those cations with high valence numbers and small CN tend not to share polyhedra with each other since this causes cations to be displaced away from shared edges • 5. (Rule 5)--Principle of Parsimony • the number of different cations, anions or anionic groups tends to be small • although elements can substitute for each other in a lattice position during mineral formation, this tends to be small
IONIC SUBSTITUTION • Ionic Substitution • the ability of ions to occupy (proxy) sites in the atomic structure of a mineral during the formation of the mineral • cation proxy on a large scale is the reason for the existence of solid solution series in mineral classification • according to ionic substitution, large or small amounts of an element can take the place in many or some of the sites usually occupied by a preferred element in the atomic structure of a mineral
• trace concentrations of cations proxying for preferred elements in the atomic structure do not appear in the chemical formula but can be the basis of important information in geologic studies • Factors influencing ionic substitution • CN • any cation that takes a position in the atomic structure in place of the preferred element must form the same CN with the anion as that of the preferred element—if the rr of a bond is borderline between two CN effects of pressure and especially temperature of mineral formation must be considered as mentioned earlier

• Al+3 can proxy for Si+4 in silicates only at high temperatures as in a magma because Si always will form a CN of 4 with O but Al, 3 or 4 with O depending on temperature • ionic charge and electroneutrality • any cation which may proxy for a preferred cation must have the same or similar valence number--if too great a difference, the energy for substitution is too high and will not occur • if valence numbers are different and there is a proxy, to adhere to the rule of electroneutrality, a second ionic substitution involving another proxy must occur--in Na. Al. Si 3 O 8, for every Ca+2 proxying for a Na+1, there must be a corresponding Al+3 substitution for a Si+4 for Ca. Al 2 Si 2 O 8 to form

• Electronegativity • if electronegativity( numbers) between the preferred element and substituting element is too great and would result in a weaker ionic bond with the anion, the substitution will not occur • review % ionic character of a bond in respect to the differences in electronegativities between the 2 elements comprising a bond
- Slides: 19