ATOMIC STRUCTURE WJEC Chemistry Unit 1 1 2

- Slides: 10

ATOMIC STRUCTURE WJEC Chemistry Unit 1 - 1. 2 WJEC Science (Double Award) Unit 2 - 2. 2

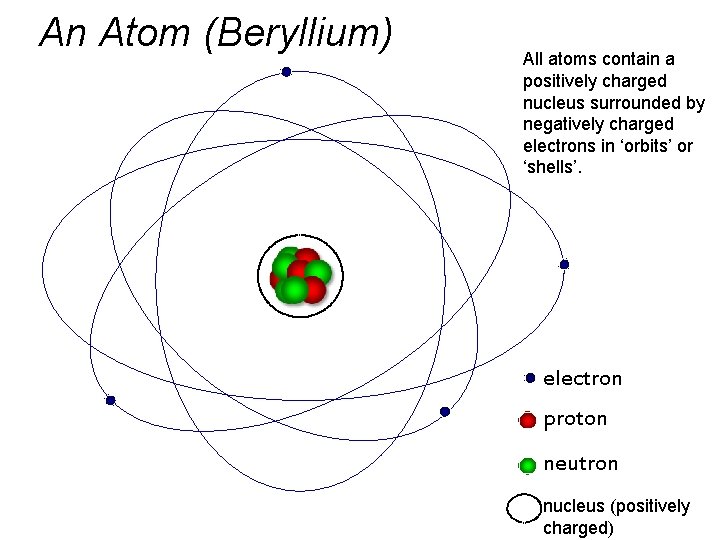
An Atom (Beryllium) All atoms contain a positively charged nucleus surrounded by negatively charged electrons in ‘orbits’ or ‘shells’. electron proton neutron nucleus (positively charged)

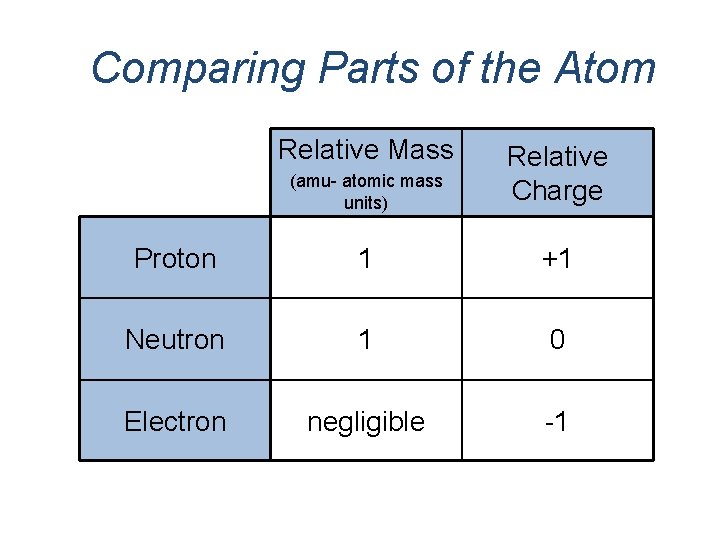
Comparing Parts of the Atom Relative Mass (amu- atomic mass units) Relative Charge Proton 1 +1 Neutron 1 0 Electron negligible -1

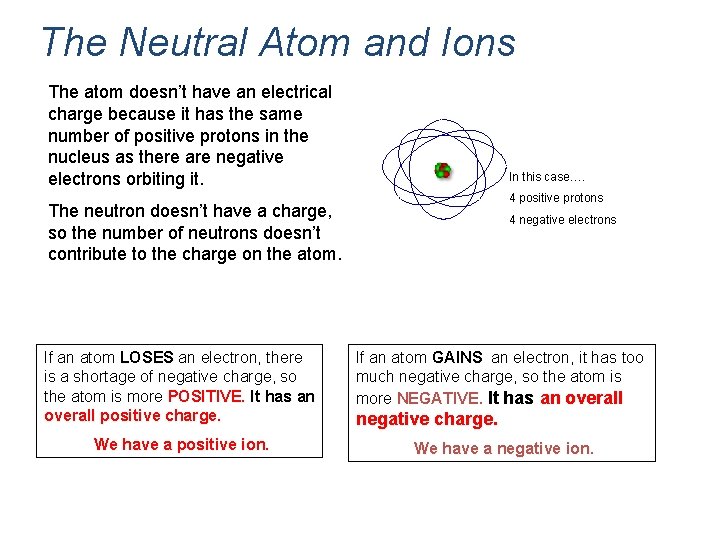
The Neutral Atom and Ions The atom doesn’t have an electrical charge because it has the same number of positive protons in the nucleus as there are negative electrons orbiting it. In this case…. 4 positive protons The neutron doesn’t have a charge, so the number of neutrons doesn’t contribute to the charge on the atom. 4 negative electrons If an atom LOSES an electron, there is a shortage of negative charge, so the atom is more POSITIVE. It has an overall positive charge. If an atom GAINS an electron, it has too much negative charge, so the atom is more NEGATIVE. It has an overall We have a positive ion. We have a negative ion. negative charge.

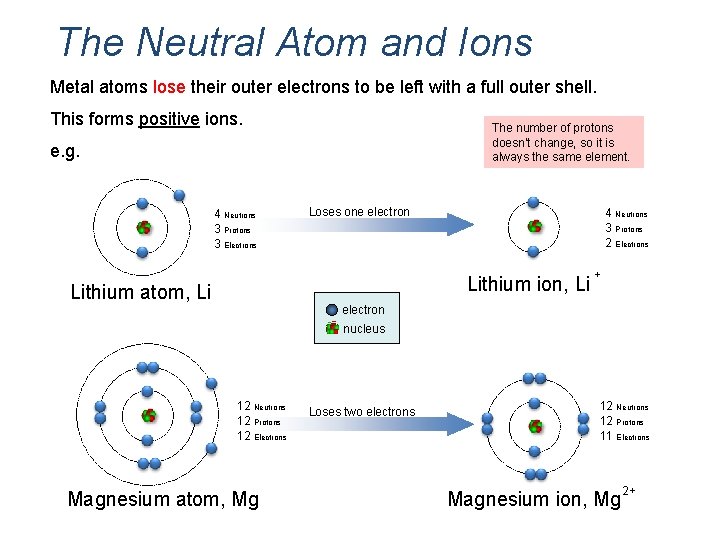
The Neutral Atom and Ions Metal atoms lose their outer electrons to be left with a full outer shell. This forms positive ions. The number of protons doesn’t change, so it is always the same element. e. g. 4 Neutrons 3 Protons 3 Electrons Loses one electron 4 Neutrons 3 Protons 2 Electrons Lithium ion, Li Lithium atom, Li + electron nucleus 12 Neutrons 12 Protons 12 Electrons Magnesium atom, Mg Loses two electrons 12 Neutrons 12 Protons 11 Electrons Magnesium ion, Mg 2+

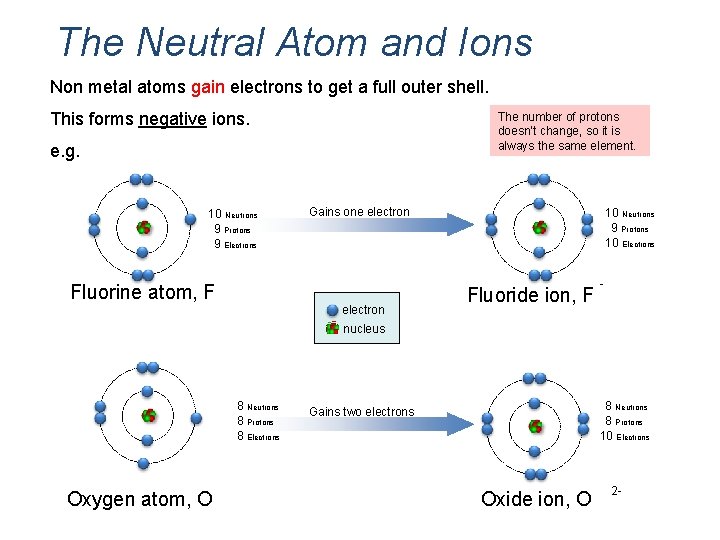
The Neutral Atom and Ions Non metal atoms gain electrons to get a full outer shell. This forms negative ions. The number of protons doesn’t change, so it is always the same element. e. g. 10 Neutrons 9 Protons 9 Electrons Fluorine atom, F Gains one electron 10 Neutrons 9 Protons 10 Electrons Fluoride ion, F - nucleus 8 Neutrons 8 Protons 8 Electrons Oxygen atom, O 8 Neutrons 8 Protons 10 Electrons Gains two electrons Oxide ion, O 2 -

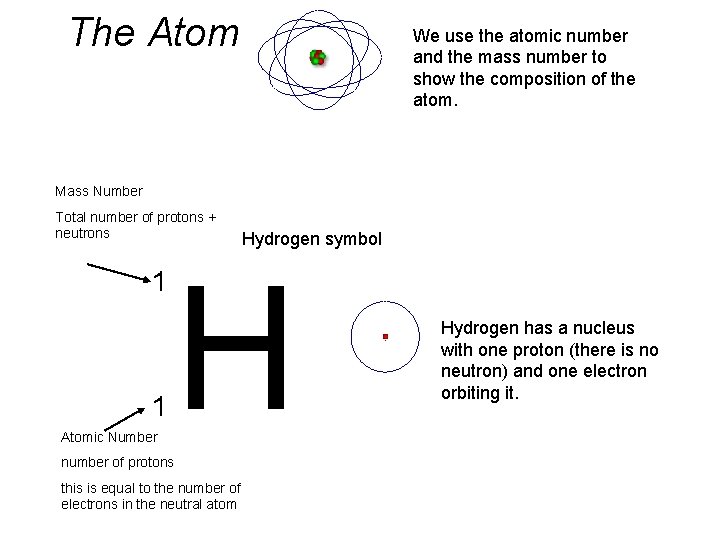
The Atom We use the atomic number and the mass number to show the composition of the atom. Mass Number Total number of protons + neutrons 1 1 Atomic Number Hydrogen symbol H number of protons this is equal to the number of electrons in the neutral atom Hydrogen has a nucleus with one proton (there is no neutron) and one electron orbiting it.

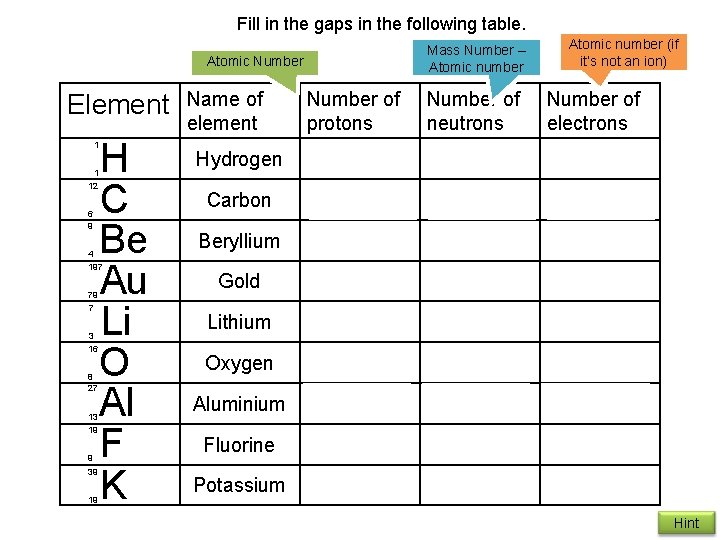
Fill in the gaps in the following table. Mass Number – Atomic number Atomic Number Element H C Be Au Li O Al F K 1 1 12 6 9 4 197 79 7 3 16 8 27 13 19 9 39 19 Name of element Hydrogen Carbon Beryllium Gold Lithium Oxygen Aluminium Fluorine Potassium Number of protons 1 6 4 79 3 8 13 9 19 Number of neutrons 0 6 5 118 4 8 14 10 20 Atomic number (if it’s not an ion) Number of electrons 1 6 4 79 3 8 13 9 19 Hint

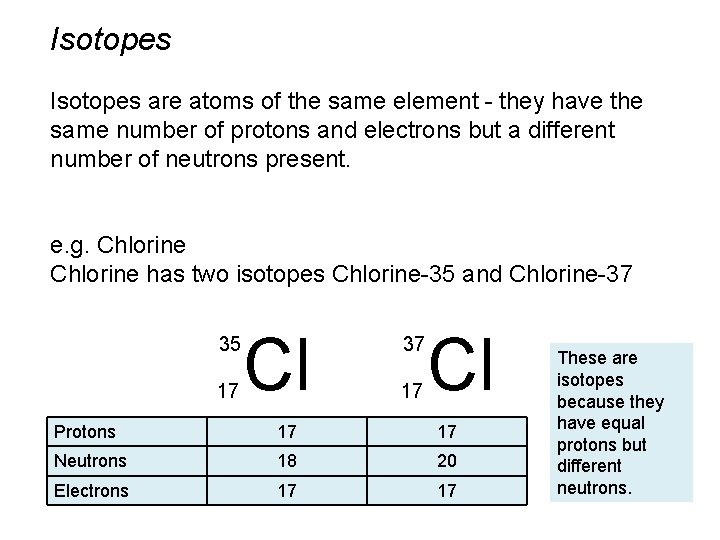
Isotopes are atoms of the same element - they have the same number of protons and electrons but a different number of neutrons present. e. g. Chlorine has two isotopes Chlorine-35 and Chlorine-37 35 17 Cl 37 17 Cl Protons 17 ? Neutrons 18 ? 20 ? Electrons 17 ? These are Why are these isotopes? because they have equal protons but different neutrons.

Relative Atomic Mass (Ar) As different elements are made up of different numbers of isotopes, their relative atomic mass is often shown, which is the average value for the isotopes of that element (this is rarely a whole number). For example: A sample of chlorine contains 25% chlorine-37 and 75% chlorine-35 Ar (Cl) = (% x isotope mass) + (% x isotope mass) 100 Ar (Cl) = (25 x 37) + (75 x 35) 100 = 3550 100 = 35. 5